Anaemia of chronic disease

Key points
Anaemia of chronic disease is common and its diagnosis should prompt the search for an underlying systemic disorder if none is obvious
It is important to exclude other causes of anaemia, especially iron deficiency
Treatment of the underlying cause may improve anaemia, but other treatment options include erythropoietin and parenteral iron; serum ferritin is a poor predictor of response to intravenous iron
If erythropoietin is used, the lowest dose that avoids the need for blood transfusion but keeps haemoglobin at, <120 g/l should be used
Future research is likely to focus on inhibition of hepcidin, a polypeptide produced in the liver in response to inflammatory stimuli, which produces a state of ‘functional iron deficiency’ – one of the key mechanisms in anaemic of chronic disease
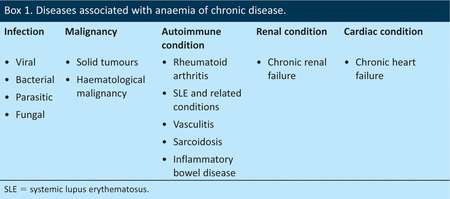
Anaemia of chronic disease (ACD), sometimes known as anaemia of inflammation, is the second most common form of anaemia worldwide1 and is seen in a variety of conditions, including cancer, autoimmune conditions and infections (Box 1). Recent years have seen significant advances in understanding the pathogenesis of ACD, and the aim of this article is to briefly review these and to provide a practical guide to the diagnosis and management of ACD.
Diseases associated with anaemia of chronic disease
Pathogenesis of ACD
The conditions associated with ACD share features of acute or chronic immune activation, with immune mechanisms significantly contributing to the observed anaemia. Indeed, anaemias associated with other conditions such as heart failure, old age and obesity may also be caused, at least in part, by subtle proinflammatory changes.2–4
The low serum levels of iron usually seen in patients with ACD are now known to be mediated by the polypeptide hepcidin, which is produced in the liver and plays a key role in iron balance and transport.1 Production of hepcidin is increased by inflammatory cytokines, particularly interleukin (IL) 6, as well as in conditions of iron overload,1 whereas levels reduce in iron deficiency. Hepcidin acts by binding to ferroportin and blocking iron export from macrophages and hepatocytes. At the same time, iron absorption by duodenal enterocytes is downregulated. The combined effect is to produce a state of ‘functional iron deficiency’ (FID) (Fig 1).
Mechanisms of anaemia in anaemia of chronic disease. EPO = erythropoietin; IFN = interferon; IL = interleukin; TNF = tumour necrosis factor.
In ACD, the normal response of increasing production of erythropoietin (EPO) in response to decreasing levels of haemoglobin (Hb) is blunted, possibly mediated through increased levels of IL-1 and tumour necrosis factor alpha (TNF-α). At the same time, EPO-induced proliferation and differentiation of early erythroid precursors is reduced. Finally, limited evidence supports a reduction in red cell survival in patients with ACD.
Diagnosis of ACD
Diagnosis of ACD may be relatively straightforward in a patient with an existing diagnosis of a chronic inflammatory or malignant disorder, but may be more challenging where such a condition is not known to be present. The anaemia is typically normochromic and normocytic, but it may become microcytic as the disease progresses.1 Other causes of anaemia need to be excluded,1,5 including nutritional deficiencies, haemoglobinopathies, haemolysis, hypogonadism, hypothyroidism, myelodysplastic syndrome (MDS), drug effects and recurrent phlebotomy. The reticulocyte count is low. Presence of inflammation may be inferred from leucocytosis, thrombocytosis and inflammatory markers.
Exclusion of iron-deficiency anaemia (IDA) is important in the work up of patients with ACD, although the conditions frequently co-exist: many patients with ACD have a state of FID and they may respond to intravenous iron supplementation even though body iron stores are ‘adequate’.6 Measurement of serum ferritin is of limited value, as ferritin is an acute-phase protein and levels are increased in the presence of inflammation. In patients with anaemia of chronic kidney disease (CKD), improvements in Hb levels are seen following intravenous iron, even in those with ferritin levels of 500–1,200 ng/ml.7 As a general rule, however, ferritin levels of <100 ng/ml in patients with ACD should be taken to indicate coexistent IDA and predict a high likelihood of response to iron supplementation.
Serum iron levels and transferrin saturation are decreased in patients with ACD, and transferrin levels are increased in IDA but normal or decreased in ACD. However, transferrin saturation has poor sensitivity and specificity when used in isolation. Although Perl's stain of a bone-marrow biopsy remains the gold standard for assessment of iron status, the process is invasive and of little value in diagnosis of ACD, although bone marrow biopsy may exclude other causes of anaemia such as MDS.
Assay of serum transferrin receptor (sTfR) has been proposed as a tool for distinguishing ACD from IDA,8 as levels do not vary from steady state in the former, but are increased in the latter. However, the assay is neither widely available nor standardised. The ratio of sTfR to the log ferritin value may be of more value in the diagnosis of ACD,9 with values of <1 making ACD likely, whereas ratios of >2 suggest that iron stores are deficient, with or without ACD. The new red-cell indices reticulocyte haemoglobin content (CHr) and percentage hypochromic red cells (%HYPO), which are available on some modern blood-count analysers, may also contribute to the diagnosis of ACD by providing information about iron supply to red-cell precursors and particularly may help to decide whether iron supplementation will improve haemoglobinisation in individual patients.10
Measurement of hepcidin may prove to be useful in the diagnosis of ACD; hepcidin can be assayed using mass spectrometry or immunoassay, with several assays now commercially available. Increased levels of hepcidin are found in a wide variety of inflammatory and malignant conditions, but it is important to note that levels of hepcidin fall in patients with co-existent ACD and IDA, as the inflammation-induced increase in hepcidin will be opposed by the effects of iron deficiency.6 As one of the long-term effects of hepcidin is to produce iron deficiency through suppression of intestinal iron absorption, the use of hepcidin levels to diagnose ACD will have to be evaluated carefully.
Overall, no uniform laboratory criteria for the diagnosis of ACD exist. Therefore, it may be necessary to assess several laboratory parameters together with the clinical picture when making a diagnosis (Table 1).
Use of laboratory investigations in the differential diagnosis of ACD.
Management of ACD
Anaemia can independently impact on morbidity and mortality in patients with conditions associated with ACD,11,12 as well as affecting quality of life, although whether measures to improve Hb levels in ACD will improve survival remains to be seen.13 Treatment of the underlying condition – for example, the use of steroids in polymyalgia rheumatica or TNFα inhibitors in inflammatory bowel disease – will often result in improvement of anaemia; indeed, improvements in the management of many conditions that cause ACD, including HIV and rheumatoid arthritis, mean that specific correction of anaemia is often unnecessary. However, in patients with other conditions, such as advanced cancer or cardiac failure, the degree of anaemia reflects the severity of the underlying cause, so correction may improve both prognosis and quality of life.
Blood transfusion
Transfused blood remains a precious resource and also carries risks of transfusion-transmitted infection, alloimmunisation and haemosiderosis. Blood transfusion should therefore be reserved for patients with severe and life-threatening anaemia.
Erythropoiesis-stimulating agents
The blunted EPO response and relative insensitivity of erythroid precursors to endogenous EPO make the use of erythropoiesis-stimulating agents (ESAs) a rational approach to management of ACD, and there is considerable experience with these agents in the setting of renal disease, cancer and, more recently, anaemia of heart failure.14,15 Several recombinant human EPOs are available, with epoetin alfa (Eprex) and darbepoetin (Aranesp) used in the UK, alongside biosimilars such as biosimilar epoetin alfa (Binocrit).
Patients with ACD caused by cancer, rheumatoid arthritis and HIV infection frequently gain benefit from EPO. The greatest experience comes from patients with cancer, in whom there is now good evidence of improved Hb levels, a reduced need for transfusions and improved quality of life.13,14 However predicting which patients will respond to EPO is not straightforward, and it is important, as discussed earlier, to rule out concomitant iron deficiency that may limit the response to EPO.
However, concerns exist due to data showing possible adverse effects associated with ESAs – for example, cardiovascular risk, especially the risk of thrombosis, may be increased – and authorities now recommend maintaining Hb levels at ≤120 g/l to minimise this risk.16 Data also suggest an increased risk of tumour recurrence in patients with cancer who receive ESAs, and the US Food and Drug Administration recommends avoiding the use of ESAs in patients with cancers of the breast, head and neck, those with non-small cell lung cancer and those with active malignancies not receiving chemotherapy or radiotherapy,17 while the National Institute for Health and Clinical Excellence (NICE) in the UK limits use to women with ovarian cancer receiving platinum-based chemotherapy or patients who cannot receive blood transfusions,18 although a recent meta-analysis casts doubt on these recommendations.19
Parenteral iron therapy
The FID present in many patients with ACD and the frequent co-existence of true IDA and ACD make iron supplementation an attractive method for the treatment of ACD.6 Safer forms of parenteral iron have become available in recent years and are widely used in conjunction with ESAs in the management of renal anaemia. Indeed, there is mounting evidence that parenteral iron in addition to ESAs may enhance response to the latter in patients with cancer-associated anaemia.20 A recent study even hypothesised that iron supplementation may reduce the thrombotic risk associated with ESAs by reducing the platelet count.21
It is recommended that iron status is assessed periodically in patients receiving ESAs, and consideration should be given to administration of intravenous iron in patients who show suboptimal response to EPO. Limited studies show that intravenous iron given alone without ESAs may improve Hb levels in patients with ACD in association with cancer and heart failure, but further research is needed in this regard. Theoretical concerns about the increased risk of infection in patients given intravenous iron have not been borne out.
Future directions
Ongoing research is investigating the effects of inhibition of hepcidin – either through direct antibody-mediated approaches or by indirect inhibition through other small molecules in the hepcidin/ferroportin pathway. An intriguing observation is the association of ACD with vitamin D deficiency in a large population-based survey in the USA,22 although further studies will need to be performed to determine whether vitamin D supplementation can suppress inflammation and ameliorate anaemia in the setting of inflammation.
- © 2013 Royal College of Physicians
References
- ↵
- ↵
- Kleijn L,
- Belonje AM,
- Voors AA,
- et al.
- ↵
- ↵
- Bloxham E,
- Vagadia V,
- Scott K,
- et al.
- ↵
- Goodnough LT,
- Nemeth E,
- Ganz T.
- ↵
- Coyne DW,
- Kapoian T,
- Suki W,
- et al.
- ↵
- Koulaouzidis A,
- Said E,
- Cottier R,
- Saeed AA
- ↵
- Skikne BS,
- Punnonen K,
- Caldron PH,
- et al.
- ↵
- Thomas C,
- Thomas L.
- ↵
- ↵
- ↵
- Wilson J,
- Yao GL,
- Raftery J,
- et al.
- ↵
- Littlewood TJ,
- Bajetta E,
- Nortier JW,
- et al.
- ↵
- Ngo K,
- Kotecha D,
- Walters JA,
- et al.
- ↵
- Rizzo JD,
- Brouwers M,
- Hurley P,
- et al.
- ↵US Food and Drug Administration. Erythropoiesis-stimulating agents (ESAs). Silver Spring, MD: FDA, 2007. www.fda.gov/NewsEvents/Testimony/ucm110908.htm [Accessed 1 February 2013].
- ↵National Institute for Health and Clinical Excellence (NICE). Erythropoetin (alfa and beta) and darbepoetin for the treatment of cancer-treatment induced anaemia. London: NICE, 2009. www.nice.org.uk/nicemedia/live/11990/40744/40744.pdf [Accessed 27 February 2013].
- ↵
- ↵
- ↵
- Henry DH,
- Dahl NV,
- Auerbach MA
- ↵
- Perlstein TS,
- Pande R,
- Berliner R,
- Vanasse GJ
Article Tools
Citation Manager Formats
Jump to section
Related Articles
- No related articles found.










