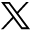Acute oesophageal symptoms

ABSTRACT
Acute oesophageal symptoms include acute dysphagia or food bolus impaction (most commonly due to strictures, Schatzki ring and eosinophilic oesophagitis), acute chest pain with odynophagia due to oesophageal infections, motility disorders and acute oesophageal rupture (of which oesophageal intramural haematoma is a subtype). Acute full thickness oesophageal rupture carries a high mortality if not recognised early; the clinical features and conditions with which this may be confused are presented and discussed.
Differentiate dysphagia from globus and early satiety.
Food bolus obstruction occurs commonly with oesophageal strictures, Schatzki rings and eosinophilic oesophagitis.
Eosinophilic oesophagitis may present with normal endoscopy and requires biopsies from upper and lower oesophagus for diagnosis.
Proton pump inhibitors can effectively treat many cases of both Schatzki ring and eosinophilic oesophagitis without the need for endoscopic dilatation.
Oesophageal motility disorders rarely present with food bolus obstruction but a prior history of intermittent dysphagia for liquids and solids and normal endoscopy is frequent.
Acute oesophageal rupture needs to be diagnosed early to reduce mortality and can mimic several acute chest conditions.
Acute oesophageal symptoms: definitions and causes
This review covers presentation and management of acute dysphagia, odynophagia and oesophageal chest pain.
Dysphagia means ‘difficulty in swallowing’, distinguished from odynophagia (‘pain on swallowing’) by a sensation of bolus arrest/delay, localised anywhere between cricoid and xiphisternum. The sensation of obstruction is associated with swallowing, differentiated from globus – a ‘blockage’ or ‘lump’ between cricoid and sternal notch independent of swallowing, typically persisting for hours1 – and early satiety. The latter is often described as food ‘lying’ in the epigastrium or the retrosternal area but differing from dysphagia, which arises during swallowing, whereas the fullness of early satiety builds during a meal, eventually inhibiting intake. Food bolus obstruction is sudden-onset total dysphagia, discussed in more detail below.
Oesophageal pain resembles other intra-thoracic/upper abdominal visceral pain (eg heart, aorta, gallbladder or lungs), not surprisingly since sensory pathways from these organs and chest wall enter the thoracic spinal cord at similar levels. Thus, oesophageal pain may occur in the chest, epigastrium, back, jaws or arm(s), and is more likely when dysphagia or odynophagia are present.
Common causes of acute oesophageal symptoms are listed in Box 1.
Causes of acute oesophageal symptoms.
Food bolus impaction
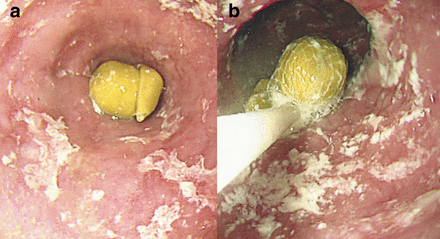
Food bolus impaction is commonest in younger adults. In total, 88–97% of all patients with food bolus impaction have underlying oesophageal abnormalities.2 Schatzki ring and peptic stricture are the commonest abnormalities2–4 (Table 1), followed by eosinophilic oesophagitis (EOE), which accounts for over 50% of food impactions in some published case series2 (Fig 1). Less common causes are achalasia (Fig 2), reflux oesophagitis, extrinsic oesophageal compression and oesophageal cancer.
Endoscopic appearances of (a) Schatzki ring, a ring at the gastro-oesophageal mucosal junction above a small sliding hiatus hernia, and (b) eosinophilic oesophagitis with rings and subtle longitudinal oesophageal furrows.
(a) Food bolus obstruction lasting 36 hours due to a pea in an elderly patient with known achalasia and (b) removal of the pea with a Roth net. Following removal the patient was treated with 80 U botulinum toxin into the tight lower oesophageal sphincter.
Food bolus impactions are acute events immediately recognised by sufferers. Impactions at cricoid level may be accompanied by choking, whereas impaction in the lower oesophagus may be perceived in the upper chest or base of neck. Typically during a meal containing meat, patients report a sudden inability to swallow solids or liquids (even saliva).The impacted bolus is regurgitated or passes on spontaneously after minutes or hours, but 10–20% remain impacted.2 Associated features include choking, excessive salivation, neck or retrosternal pain, vomiting and retching. Episodes may recur infrequently –for years in patients with EOE or Schatzki ring –but recurrent (non-impaction) dysphagia raises the possibility of underlying benign or malignant stricture, the latter typically in older patients with a shorter history of progressive dysphagia. Intermittent dysphagia for liquids and solids suggests a motility disorder. A history of atopy or allergy may accompany EOE.
Patients with recurrent food bolus obstruction learn corrective measures, eg carbonated drinks, or manoeuvring the neck or torso into particular positions. During unresolved impaction, airway patency and risk of aspiration should be assessed. Fever, tachycardia, and/or surgical emphysema in the neck suggests perforation.
Management
Plain radiography rarely helps as most food is radiolucent, but chest X-ray may suggest perforation (pneumomediastinum); contrast radiography risks aspiration. Patients without complications should undergo endoscopy, the timing of which depends on clinical features. Excessive salivation and aspiration risk require emergency endoscopy; otherwise endoscopy should be performed within 6–12 hours. Glucagon 1.0 mg intravenously (to relax oesophageal smooth muscle) has been advocated to allow spontaneous passage of impacted food. Though studies show success rates of 12–50%, a randomised trial failed to substantiate this.2 The food bolus can be extracted endoscopically using a Roth net, en bloc or piecemeal (Fig 2), or gently pushed into the stomach, sometimes after breaking it up with a wire snare. In two studies of 375 patients with impaction, the push technique was successful in 84–97% with no perforations.3–5 Endoscopy may detect Schatzki ring or peptic stricture, and allow endoscopic dilation, though in both (unless the stricture is severe) improvement often occurs with proton pump inhibitors (PPIs).
Endoscopy may show features of EOE (oesophageal rings, linear furrows (Fig 1) and mucosal fragility), but may be normal in 20–30%; two biopsies should be taken from the distal and upper oesophagus even with normal endoscopy. A histopathological finding of ³15 eosinophils per high power field is diagnostic.6 In total, 30–40% of EOE patients respond to PPIs;7 the remainder require repeated courses of swallowed topical steroids (eg budesonide or fluticasone) or systemic steroids.8 Hypoallergenic diets (elemental and elimination) are also effective.9
Oesophageal dysmotility
In the context of acute chest pain and dysphagia, oesophageal motility disorders should be considered when endoscopy and oesophageal biopsies are normal. Food bolus obstruction occurs rarely with achalasia (Fig 2) or oesophageal spasm, usually on a background of intermittent dysphagia and regurgitation for liquids and solids.1,2 Achalasia symptoms may be severe enough to cause weight loss, whereas oesophageal spasm presents with intermittent chest pain (often resembling angina) and dysphagia. While barium-swallow radiology and endoscopy may show characteristic appearances for achalasia (dilated oesophagus with food residue, tight lower oesophageal sphincter), these may be normal.
Modern diagnosis relies on high-resolution manometry with specific metrics and an algorithm (Chicago classification)10 categorising motility disorders into disorders of oesophagogastric outflow obstruction (including three achalasia variants), major disorders of peristalsis (including oesophageal spasm) and minor peristaltic disorders (see Fig 3).
High-resolution oesophageal manometry with topographical maps of oesophageal motility. X axis shows time (10-second intervals), Y axis shows oesophageal length with UES as a continuous bar above and LES below. Both sphincters relax with swallowing. The colours indicate pressure (blue-green lowest, red-purple highest. (a) Normal; (b) achalasia with non-peristaltic pan-oesophageal pressurisation and failed/incomplete LES relaxation; and (c) diffuse spasm – some non-peristaltic waves, a high pressure prolonged duration wave, normal LES relaxation. LES = lower oesophageal sphincter; TZ = transitional zone (aortic impression); UES = upper oesophageal sphincter.
Management of achalasia and oesophageal spasm
Treatment of achalasia is by lowering lower oesophageal sphincter pressure. Pharmacotherapy has a limited role; however, sublingual nifedipine may give short-term relief. Botulinum toxin injection into the lower oesophageal sphincter is effective in 85% cases, but effects diminish with time; only 30% remain improved at 2 years.11 Repeated botulinum injection is useful for those unfit for surgery.11 Others can be offered graded 30–40 mm pneumatic balloon dilation (2–3% risk of oesophageal perforation) or laparoscopic Heller's myotomy. Dilation and surgery have comparable success rates,12 and therefore choice depends on patient preference and institutional expertise. Peroralendoscopic myotomy (POEM) is a new endoscopic procedure but experience is currently limited.13
For oesophageal spasm, diagnose and treat gastro-oesophageal (GO) reflux (if present). If GO reflex is absent, smooth muscle relaxants may work if given with symptoms (eg glyceryltrinitrate spray or sublingual nifedipine), but regular dosing is less effective. Low-dose tricyclic agents (eg imipramine 50–100 mg at night) may reduce chest pain, though the use of agents is often limited by side effects. Other options include botulinum toxin into the distal 10 cm of the oesophageal wall (for predominant dysphagia symptoms),14 sildenafil15 or rarely, surgical long myotomy or POEM.16
Oesophageal infection
Infectious oesophagitis presents with acute odynophagia and chest pain, sometimes with fever and vomiting.17 The commonest agent is Candida albicans. Two other important organisms are cytomegalovirus (CMV) and herpes simplex virus (HSV). All may occur with impairment of normal physiology (salivation and oesophageal motility), but most commonly with immunosuppression (eg HIV, diabetes, malnutrition, cancer or immunosuppressant use). HSV can occur in young immunocompetent individuals.
Oesophageal candidiasis often presents without oral thrush, especially among patients using inhaled steroids. Endoscopy is usually needed for diagnosis. Management should be with systemic antifungals (eg fluconazole 50–100 mg daily for 1–2 weeks).17
Endoscopic appearances of circumscribed punched-out oesophageal ulcers with raised edges are characteristic of HSV oesophagitis, confirmed by biopsies showing multinucleate giant cells, with ground-glass nuclei and eosinophilic inclusions. Aciclovir is the treatment of choice.17 CMV oesophagitis appears as large (often linear) deep ulcers in the mid or lower oesophagus with biopsies showing intranuclear/intracytoplasmic inclusions. Ganciclovir is the treatment of choice.17
Spontaneous oesophageal rupture
First described in 1724 by Herman Boerhaave, whose name is eponymous with the condition, spontaneous rupture of the oesophagus occurs with sudden increases in intraoesophageal pressure with negative intrathoracic pressure. It is part of a spectrum including Mallory–Weiss tear and oesophageal intramural haematoma.18 15% of oesophageal perforations are spontaneous with a prevalence of 3.1/1000000/year.19 This rare condition carries a mortality of approximately 35%, but a delay in diagnosis of 12–24 hours increases mortality to over 60%.19 Forceful vomiting is the commonest cause, followed by seizures, prolonged coughing, weight lifting, straining at defecation, food binging and laughing.
Mackler's diagnostic triad includes vomiting, chest pain and subcutaneous emphysema, but some of these classic features may be absent. Severe chest pain, often radiating to the back, is typical, though occasionally absent. Subcutaneous emphysema takes at least one hour to become apparent. Other features include odynophagia, tachycardia and tachypnoea. Early diagnosis is likeliest if the possibility is entertained; differential diagnoses include myocardial infarction, pulmonary embolism, pneumonia and aortic dissection – 30% are initially mis-diagnosed.20 Chest X-ray may show pneumomediastinum and/or pleural effusion (often left sided), but may be normal; computerised tomography(CT) chest scan with oral and intravenous contrast allows precise diagnosis.
Management includes early review by an oesophagogastric surgeon to consider emergency operative repair or oesophagectomy; established intrathoracic contamination/sepsis with delayed diagnosis requires antibiotics/antifungals, drainage of sepsis and sealing of the perforation with a covered removable oesophageal stent21 or more recently by endoscopic clips.22 This may be best for patients unfit for surgery, who require intensive care unit management and parenteral feeding.
Oesophageal intramural haematoma
Oesophageal intramural haematoma has been reported to occur most frequently among patients with coagulopathies or using anticoagulants or antiplatelet agents; it may also occur spontaneously, or as a result of a sudden increase in intra-oesophageal pressure, or following endoscopic procedures. It presents with acute chest/back pain, dysphagia/odynophagia and haematemesis. Diagnosis is by CT scan showing a non-enhancing oesophageal mass, and/or endoscopy showing a smooth bluish oesophageal mucosal bulge.18 Management is supportive, including correcting clotting disturbances (if present). The oesophageal mucosa overlying the haematoma usually sloughs off within 2–3 weeks, leaving a long linear ulcer.18
Concluding remarks
Acute oesophageal symptoms require a careful history: dysphagia may be confused with globus and with early satiety. Acute food bolus obstruction is readily recognised but the underlying causes may be subtle (Schatzki ring, eosinophilic oesophagitis or motility disorders) and easily missed at endoscopy. Acute chest pain and odynophagia may be due to motility disorders and oesophageal infections, but acute oesophageal rupture and a less severe variant (oesophageal intramural haematoma) are easily overlooked if not considered early in the differential diagnosis of acute chest pain.
- © Royal College of Physicians 2015. All rights reserved.
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- Sawas T
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- Talukdar R
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- Blencowe NS
- 20.↵
- 21.↵
- 22.↵
Article Tools
Citation Manager Formats
Jump to section
Related Articles
- No related articles found.
Cited By...
- No citing articles found.