Reducing inappropriate blood testing in haematology inpatients: A multicentre quality improvement project

ABSTRACT
Haematology inpatients are subject to extensive blood testing and many of these tests could be deemed inappropriate as they are not indicated for monitoring or clinical symptoms. Unnecessary testing exposes the patient to the risks of phlebotomy and adds resources’ strain to the NHS.
Our aim was to reduce the number of inappropriate blood tests performed on haematology inpatient wards.
Quality improvement projects (QIPs) were performed in four haematology units introducing inpatient blood testing schedules (BTS) or providing staff education on current schedules.
A reduction in inappropriate or overall blood testing was achieved at every site where a BTS was implemented, with a median reduction in inappropriate blood testing of 24.7% and estimated cost savings of up to £38,438 per annum.
This QIP can be safely adapted to a variety of inpatient settings and is associated with cost savings. This initiative could be extended to other inpatient departments throughout the NHS.
Introduction
Problem
It is common practice for haematology inpatients to be subject to daily blood testing, which was observed at all four centres participating in this quality improvement project (QIP).
While haematology patients are likely to require frequent phlebotomy for monitoring high-intensity chemotherapy or bone marrow transplant (BMT), the frequency of testing is rarely dictated by guidelines, but rather by clinical expertise. When the person ordering the blood tests is the most junior team member, the default is often multiple daily blood tests. In addition, there may be other frequent testing trends that have become embedded in the department, for example lactate dehydrogenase (LDH). However, many of these tests are inappropriate (IA) as they are not clinically indicated, or the frequency is excessive.
In an NHS where both human and financial resources are facing excessive demand, a reduction in inappropriate phlebotomy episodes and laboratory blood tests has the potential to save money and staff time. It also reduces the risk to patients from excessive phlebotomy.1
Background
Blood tests are an essential component of providing comprehensive care to inpatients with haematological conditions but may not be indicated throughout admission for lower intensity chemotherapy and non-malignant conditions. While the omission of relevant investigations may put a patient at risk, the overuse of tests that are not indicated can lead to increased patient morbidity and increased financial outlay.
It is estimated that 28% of blood tests are taken inappropriately, which comes at a significant cost to patients and healthcare systems.2 Phlebotomy is both invasive and painful to patients and, in addition to this, medical complications can arise due to excess blood taking, including hospital-acquired / phlebotomy-induced anaemia and infection in an inpatient population who are often already anaemic and immunocompromised.2,3 It is estimated that for every 80 mL of blood drawn, haemoglobin levels can fall by 1.0 g/dL.4 This is associated with increased need for blood transfusions (plus transfusion-associated risks), increased length of stay and increased mortality.1,4–6
Although laboratory testing represents a relatively small proportion of total healthcare costs, the true impact of excess blood testing is generated by its downstream costs; including further testing, and extended hospital stay.3 The cost of an unnecessary blood tests ranges from £0.23 to £3.43, while the cost of a transfusion (£170) and extended hospital stays are significantly higher.7
Proof of principle
We introduced a blood test schedule (BTS) at St Bartholomew’s hospital (SB) and demonstrated that a cost saving is associated with a reduction in the number of inappropriate blood tests taken.8 This QIP builds upon the data collected at SB and is continued at three further sites for multicentre comparison and analysis.
Aim
The aim for each site was to safely reduce the number of inappropriate blood tests in haematology ward inpatients to as near zero as possible.
Methods
Context: four haematology centres
The QIP was run at four centres consecutively between 2014 and 2018: SB 2014, Royal Oldham Hospital (ROH) 2016, St James’s University Hospital (SJUH) 2018 and Bradford Royal Infirmary (BRI) 2018. Together the centres reflect a range of haematology inpatient set-ups. SB is a specialist haemato-oncology centre in London and performs autologous and allogenic BMTs. ROH was the haematology centre for a large district general hospital trust (Pennine Acute Hospitals NHS Trust), treating malignant and non-malignant conditions but does not perform BMT. BRI is a foundation trust treating malignant and non-malignant condition and performs autologous BMT. SJUH is a teaching hospital and treats patients with malignant and non-malignant conditions and performs autologous and allogenic BMT.
Either two or three plan, do, study, act (PDSA) cycles were performed per site depending on whether a BTS was already in place, and whether sustainability was evaluated.9 At all sites, only haematology patients on haematology wards were included.
All QIPs were registered locally at the participating trust, no formal ethics approval was required.
Intervention: blood test schedule design
The teams consisted of junior doctors who collected the data and designed BTSs bespoke to each site with consultation from senior doctors and the multidisciplinary team (MDT). The consultation process outline and BTS designs are available in the supplementary material S1.
All sites included full blood count (FBC), urea and electrolytes (U&Es), liver function tests (LFTs), bone profile, C-reactive protein (CRP) and coagulation screen as routine blood tests. Other tests were included on site-specific BTSs if they were being done regularly or were necessary tests for the BTS eg cytomegalovirus (CMV) monitoring in post-allograft patients at SJUH.
SB and ROH had a pre-designed BTSs and, therefore, whether a test would be IA could be documented at baseline (cycle 1) enabling estimation of the potential change with BTS introduction.
SJUH non-allograft patients did not have a BTS proposed at baseline, therefore the assessment of whether a test was appropriate was not done in cycle 1. Unlike other sites the SJUH inpatient caseload is very heterogenous with multiple specialist teams covering inpatients. To reduce bias in BTS design the baseline results from cycle 1 were presented at the monthly management meeting with a follow-up e-mail consultation period. The result was two non-allogenic transplant inpatient BTSs (‘high-intensity chemotherapy and autografts’ and other ‘non-allogenic transplant’; supplementary material S1). When consultants disagreed on the frequency of certain test (eg CRP), the highest frequency suggested was used for the BTS.
The introduction of BTSs at all sites was accompanied by education of the ward teams and placing schedules in an accessible place for reference.
At SJUH, one of the ward clerks created a printable ‘blood test requesting sheet’ for each patient with the blood tests pre-ticked in accordance with the BTS that the patient was on. Boxes were left free for additional routine and specialist tests to be added. This approach was well received and rolled out to all (three) participating wards.
BRI inpatients and SJUH allogenic-transplant patients already had BTSs in place and, therefore, started on PDSA cycle 2.
Measurement
Blood tests completed for each inpatient were recorded over consecutive 2-week periods and labelled as ‘appropriate’ or ‘not appropriate’ by the junior doctor recording the data. Tests were appropriate if they were either on the BTS or clinically indicated. The decision process for whether a blood test was appropriate is outlined by the flowchart in supplementary material S2. The data was collected from electronic blood results and only the first set of blood tests done each day were counted as the ‘routine’ test (indications were checked); it was assumed that all subsequent testing was ‘appropriate’, as this would have to be requested during the day for a patient-specific indication.
All sites recorded the absolute number of blood tests, and SJUH also recorded the number of inpatient days. This allowed for variation in bed occupancy to be taken into account (a limitation from the previous sites) and reported as the ratio of test:inpatient days (per week). This approach allowed the frequency of testing to be effectively communicated during BTS design process. For example, at baseline LFTs had a test:inpatient days ratio of 0.96 (7/7 days = 1; 6/7 days = 0.86), which highlighted this test being done almost every day, although the consultants thought the frequency should be nearer three times per week (a ratio of 0.43).
PDSA cycle 1: baseline data collection
The aim for PDSA cycle 1 was to acquire baseline data on the quantity of routine blood tests before a BTS was implemented. Sites that performed PDSA cycle 1 were SB, ROH and SJUH (excluding allogenic transplant patients).
A 2-week retrospective period was evaluated by recording the number of routine blood tests and, at SB and ROH, recording whether these would be IA according to the proposed BTS.
PDSA cycle 2: schedule implementation
All centres participated cycle 2. The aim was to determine whether the BTS had an effect on the rate of IA blood testing or overall number of tests performed, and whether this translated to cost savings.
This was the first cycle for BRI and SJUH allogenic-transplant patients, who already had BTSs in place.
Data were collected prospectively for 2 weeks after the BTS had been in place for at least 1 week to allow staff to get used to it. From BTS implementation, an author was named as a point of contact for any safety concerns to be raised to by the MDT.
PDSA cycle 3: schedule evaluation
Sites ROH, BRI and SJUH (allogenic transplant) performed cycle 3. The aim was to evaluate whether the new BTS (ROH) or pre-existing BTS (BRI, SJUH allogenic transplant) retained the effect on the number of IA tests. A sustained or continued reduction in the number of IA tests would demonstrate the sustainability of the schedule effect on IA testing.
Each site had one session of education for blood test requesters prior to cycle 3, which took place at least 3 months after cycle 2 and was performed prospectively over a 2-week period.
Improving methods at subsequent sites
Effective practices (such as accessible placement of the reference BTSs) were utilised at subsequent sites. Less effective practices (such as the data recording method) was improved between sites. For example, ROH used a filled table per patient to record data, then the information had to be collated. Therefore, at SJUH, a macro-enabled excel spreadsheet was used to record the data from all patients and automatically calculate the total number of tests and test:inpatient days ratio. This enhanced the efficiency of data collection, and a similar approach was used at BRI.
Results
The baseline results for each site were SB 2,534 tests (45.9% IA); ROH 586 tests (19.1% IA); SJUH 1,012 tests (assessment of IA baseline not applicable as the results were used to inform the schedule design); BRI (schedule already in place) 845 tests (7% IA).
The upper and lower number of blood tests evaluated across the four sites ranged from 586 (ROH) to 2,534 (SB) in cycle 1; 845 (BRI) to 2,125 (SJUH) in cycle 2; and 586 (ROH) to 637 (BRI) in cycle 3.
No significant safety concerns were reported at any site.
Interestingly, all sites showed a similar level of IA testing post-BTS introduction: SB 19.6%; ROH 8% in cycle 2 and 10% in cycle 3; SJUH 7.8%; and BRI 7% in cycle 2 and 13.8% in cycle 3.
Fig 1 shows the change in IA results per test type at each site. Fig 2 demonstrates the change in the tests:inpatient days ratio per test type at SJUH.
Percentage change in inappropriate blood test requesting by test type for plan, do, study, act cycles 1–3. a) St Bartholomew’s hospital. b) Royal Oldham Hospital. c) Bradford Royal Infirmary. Coag = coagulation; CRP = C-reactive protein; FBC = full blood count; LDH = lactate dehydrogenase; LFT = liver function tests; Mg = magnesium; U&E = urea and electrolytes.
Change in the number of tests per inpatient days for plan, do, study, act cycles 1–3 at St James’s University Hospital. a) Non-allogeneic transplant schedules (‘intensive chemotherapy and autografts’ and ‘non-allogeneic transplant’). b) Allogeneic transplant schedules. Coag = coagulation; CRP = C-reactive protein; EBV/CMV = Epstein–Barr virus / cytomegalovirus; FBC = full blood count; LFT = liver function tests; Mg = magnesium; U&E = urea and electrolytes.
The estimated cost savings associated with a reduction in inappropriate blood testing was £38,438 (SB), £5,172 (ROH) and £3,400 (SJUH) per annum.
Fig 3 summarises the change in percentage of inappropriate tests for SB, ROH and BRI. SJUH used the test:inpatient days ratio to analyse data, so is not included in this figure. At SJUH, the average ratio decreased from 0.77 to 0.66 for non-allograft patients. During cycle 3 at ROH testing was increased from 8% IA to 10% IA but this difference was not statistically significant (p=0.17). The rest of the changes in IA testing rate between the first and second cycle were statistically significant (p<0.01; calculated using Fisher’s exact method).
Summary of the overall percentage inappropriate tests at St Bartholomew’s hospital, Royal Oldham Hospital and Bradford Royal Infirmary. Barts = St Bartholomew’s hospital; BRI = Bradford Royal Infirmary; ROH = Royal Oldham Hospital.
Site-specific factors affecting results
CRP testing increased at SJUH due to a consultant advising daily CRP on the BTS, although this was not practised before the BTS implementation.
BRI already had a BTS in place, and no actual cost savings were identified during the QIP. Although staff training about the BTS took place between PDSA cycles 2 and 3, the number of tests requested increased in cycle 3. This was because there was a locum doctor who was not aware of the BTS covering inpatients on the second weekend of cycle, which led to an increase in blood test requesting that skewed the data. If BRI eradiated inappropriate testing they could save £350 (lower estimate) to £1,521 (upper estimate). These low values in comparison to other sites demonstrate the efficacy of the original BRI BTS.
Estimating cost
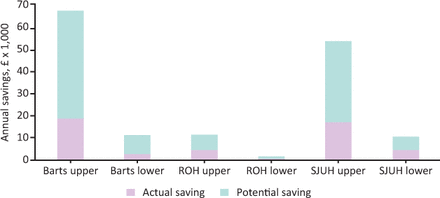
The cost of tests varied according to the test type and the site. The range was £0.23 to £3.43. SJUH costed the lowest value per test overall and SB costed the highest. Fig 4 takes this into account and presents the highest and lowest estimated savings per site if saving were calculated as per the lowest and highest site costings. The large variation in savings estimates is due to department size and how the blood tests were costed.
Upper and lower savings per site, actual and potential (if no inappropriate tests were done). Barts = St Bartholomew’s hospital; ROH = Royal Oldham Hospital; SJUH = St James’s University Hospital.
Discussion
Summary
The introduction of a BTS was associated with a reduction in inappropriate blood tests at sites where there previously was no schedule, and ROH, BRI and SJUH allografts demonstrated that a low percentage of IA tests can be sustained. The reduction in IA blood tests was associated with significant cost savings. No adverse clinical events or treatment delays occurred as a result of streamlined blood test requests.
Interpretation
A BTS was an effective intervention at all sites to reduce or sustain lower rates of IA tests than at sites without a BTS. Blood test requester education may also have helped lower IA testing rate and was well received by staff.
Achieving this outcome across four sites with a good range of haematology inpatient system models demonstrates the adaptability of the QIP design and the flexibility of the intervention.
The method could be readily generalisable to other specialist inpatient wards.
Lessons and limitations
Elements from the four sites that worked well included placing the BTS summaries in obvious places (eg doctor’s office) and educating staff about the BTS. MDT involvement was also crucial for success in both the design and implementation of the BTS. At SJUH, involvement of the ward clerks (in particular, the clerk who made the BTS sheets) was especially successful, making it easy and consistent for ward staff to use.
The use of the tests:patient days ratio worked well at SJUH to inform BTS design by presenting the average frequency of tests (eg LFTs) and prompting discussion regarding whether this could be safely decreased, and the frequency of CRP testing remains under review.
Confounding bias (such as the impact of a locum skewing the blood testing rate over a weekend in BRI cycle 3) can influence results. The prospective model after cycle 1 allowed data collectors to become aware of influences (such as locums), which was taken into account when evaluating results.
Investigator bias could have arisen from the subjectivity of whether a test was ‘appropriate’ or not. To minimise this, the data collecting author was briefed regarding what makes a test ‘appropriate’ as per the decision tool in (supplementary material S2).
The actual impact of the QIPs on service costs was challenging to measure because quotes from the laboratory for blood testing costs varied widely across the sites, as shown in Fig 3. Blood testing quotes depended on how a service was charged to a department, and whether an estimate of the whole process cost or just the laboratory staff time and reagents are taken into account. We may have underestimated the true savings from this QIP as staff time, phlebotomy consumables and waste disposal costs are not accounted for in our estimates.
In 2020, BTSs remain in use at SJUH and SB. The BRI schedule remained in place until the doctors’ office was re-organised during the first COVID-19 peak and the BTS was removed. The ROH schedule was also removed from the office and is no longer in place. This highlights the placement of the schedule for staff use as crucial for sustainability of the intervention.
Ongoing evaluation of the intervention at sites continuing and re-instating the BTS would be beneficial, including longer monitoring periods for smaller cohorts (such as allogenic transplant patients at SJUH) to provide more data.
Conclusion
A reduction in inappropriate or overall blood testing was achieved at every site where a blood test schedule was introduced, this was associated with significant cost reductions, and no adverse clinical events occurred. The sustainability of the QIPs were due to the ease of access to the schedule.
This successful initiative could also be extended to other haematology inpatient departments, or other specialist inpatient departments throughout the NHS.
Supplementary material
Additional supplementary material may be found in the online version of this article at www.rcpjournals.org/clinmedicine:
S1 – Consultation models and blood test schedules.
S1 – Tool for deciding whether a blood test is appropriate or not.
Acknowledgements
Thanks to Bethany Hughes, ward clerk at SJUH WHO produced the SJUH inpatient blood test request charts, and clinical/educational supervisor support from Allameddine Allameddine (ROH), Richard Kelly (SJUH) and Sam Ackroyd (BRI), and support from Rod Johnson, Quentin Hill and Maria Gilleece for schedule design and implementation support at SJUH.
- © Royal College of Physicians 2021. All rights reserved.
References
- ↵
- ↵
- Jalbert R
- ↵
- World Health Organization
- ↵
- ↵
- ↵
- Ko A
- ↵
- NHS Improvement
- ↵
- Akhtar W
- ↵
- ACT academy
Article Tools
Citation Manager Formats
Jump to section
Related Articles
- No related articles found.
Cited By...
- No citing articles found.