Nipah virus, an emerging zoonotic disease causing fatal encephalitis

ABSTRACT
Nipah virus is an acute febrile illness that can cause fatal encephalitis. It is an emerging zoonotic paramyxovirus endemic to south-east Asia and the western Pacific, and can be transmitted by its primary reservoir of fruit bats, through intermediate animal vectors and by human-to-human spread. Outbreaks of Nipah virus encephalitis have occurred in Malaysia, Singapore, Philippines, India and Bangladesh, with the most recent outbreak occurring in Kerala, India in late 2021. Extremely high case fatality rates have been reported from these outbreaks, and to date no vaccines or therapeutic management options are available. Combining this with its propensity to present non-specifically, Nipah virus encephalitis presents a challenging diagnosis that should not be missed in patients returning from endemic regions. Raising awareness of the epidemiology, clinical presentation and risk factors of contracting Nipah virus is vital to recognise and manage potential outbreaks of this disease in the UK.
Key points
Nipah virus is a zoonotic disease which can cause severe and fatal encephalitis..
It is endemic to south east Asia and the western Pacific, with outbreaks occurring in Malaysia, Singapore, Philippines, India and Bangladesh.
It can present non-specifically, but neurological symptoms are often common; uniquely it can cause a late-onset and relapsing encephalitis.
Risk factors for transmission include contact with its reservoir host of fruit bats, contact with known intermediate hosts such as pigs or contact with known Nipah virus patients.
No licenced medications or vaccines are currently available, with prevention and education being key to controlling outbreaks of this pathogen with pandemic potential.
Introduction
Emerging zoonoses causing neurological infections are expanding globally and clinicians should always consider pathogens exotic to the UK in returning travellers. A particular diagnosis not to miss is that of Nipah virus (NiV), a viral infection endemic to south-east Asia and western Pacific which can cause severe and fatal encephalitis.1
NiV is an RNA virus belonging to the family of Paramyxoviridae and was first isolated in 1999 after an outbreak of viral encephalitis among pig farmers in Malaysia.2 The Malaysian outbreak occurred following a spill-over event whereby NiV spread from fruit bats, its primary reservoir, to pigs and was subsequently transmitted to humans.3 It bears similarity to Hendra virus, another paramyxovirus which can cause encephalitis. Unlike Hendra virus, however, human-to-human transmission has been reported in NiV and is classed as an airborne high-consequence infectious disease in the UK.4
In recent years, outbreaks of NiV encephalitis have occurred in Bangladesh, Malaysia, Singapore, Philippines and India with high case fatality numbers. The wide distribution of its natural host, combined with NiV's potential for human-to-human spread and the paucity of therapeutic options, highlight the concern that it may have the potential to cause a global pandemic.5
Clinicians should consider NiV as a diagnosis in a returning traveller from endemic regions with reference to its epidemiology and clinical presentation, as well providing preventative advice to those travelling to at-risk locations.
Clinical presentation
Encephalitis commonly presents as a febrile illness associated with neurological changes, including convulsions, weakness, confusion and neuropsychiatric symptoms. Patients can also present with vague symptoms and case reports of NiV outbreaks illustrate how most patients presented non-specifically, with fever, headache, myalgia, respiratory and gastrointestinal symptoms being common.6–9 In particular, outbreaks in Bangladesh and India illustrate more frequent and severe respiratory involvement compared to Malaysian outbreaks.10 Neurological symptoms are common throughout all reported case series, with the majority of patients presenting with a reduced level of consciousness and prominent neurological symptoms including signs of brainstem dysfunction, behavioural disturbances, myoclonus and seizures.7 The incubation period after exposure to NiV can range from a few days to months, with an average of <2 weeks.6,7 Once inside the central nervous system (CNS), gross pathology illustrates that NiV causes neuronal damage through a dual mechanism of vasculitis- induced disseminated thrombosis and direct neuronal infection.11 This leads to a severe encephalitis which can either develop acutely or sub-acutely as a late-onset encephalitis (the latter being unique to NiV and difficult to diagnose due to exposure potentially occurring months earlier). A further distinctive clinical syndrome of relapse encephalitis has been reported in cases of NiV, with encephalitis resurging several months to years after recovering from the symptomatic initial infection.12 A case series of 160 patients showed more than 10% of NiV patients suffered with either a relapse of their encephalitis or late-onset encephalitis, illustrating the importance to elucidate a detailed travel and exposure history spanning more than ‘recent travel’.13 The clinical features of both late-onset and relapse NiV encephalitis are similar to the acute episode of encephalitis, with seizures and focal neurological signs common (Table 1).
Symptomology of Nipah virus encephalitis
It is important to consider other differential diagnoses in returning travellers with encephalitis (Box 1).
Differential diagnosis
When to suspect Nipah virus – epidemiology and transmission risk factors
The Pteropus fruit bats are the natural reservoirs of NiV. They are widely distributed across the southern hemisphere, and NiV seropositivity has been found among Pteropus populations in West Africa and Madagascar, as well as south-east Asia (Fig 1).3 Following the first outbreaks of NiV reported in Malaysia and Singapore, further outbreaks have occurred in Bangladesh, India and the Philippines.2,6–9,14,15 Of note, Bangladesh has had near-seasonal outbreaks of Nipah since 2001. Returning travellers from regions endemic with NiV or areas known to house seropositive bat populations should be considered to be high risk if presenting with encephalitis.
(a) Areas where there is risk of Nipah virus transmission. (b) Nipah virus' reservoir host, the Pteropus bat, pictured in a public park in Rajasthan, India (Photo courtesy of Jakub Halun) and reproduced under a CC BY-SA 4.0 licence.
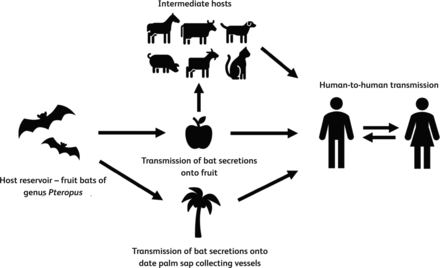
Bats harbouring NiV opportunistically forage in trees producing ripe fruit and, in Bangladesh, they often drink from date palm sap (a local delicacy) collection vessels.16 Consumption of these contaminated foods and consequent direct exposure to bat secretions can lead to disease in humans. Alternatively, contact with intermediate symptomatic host animals such as pigs, horses, dogs and cats can also result in human infection (Fig 2).3 NiV has been isolated from urine and respiratory samples of infected humans and human-to-human transmission through respiratory droplets have been reported in both household and nosocomial settings.17
Possible routes for disease transmission of Nipah virus encephalitis.
Risk factors to consider in patients presenting with encephalitis from NiV endemic regions therefore include:18,19
close contact with fruit bats (such as tree climbing or travel to rural areas) or known intermediate hosts (such as pigs)
contact with confirmed or suspected human cases of NiV
consumption of date palm sap or foraged fruits found on the ground.
Diagnosis
The preferred and most sensitive diagnostic test for NiV encephalitis is reverse transcriptase polymerase chain reaction (PCR).15 Cerebrospinal fluid (CSF), blood, nasal/throat swabs and urine samples during the acute phase of infection can be used for PCR testing for NiV.
Biosafety level 4 laboratory facilities are required for NiV particle isolation and propagation and currently no rapid diagnostic tests for NiV are available, with most testing occurring at central laboratories in endemic countries.16 In the UK, the Rare and Imported Pathogens Laboratory in Porton Down is the designated centre for running NiV PCR.20 This presents a challenge for surveillance, as screening for possible NiV cases may take several days or weeks after sample collection.
Thrombocytopenia, leukopenia and deranged liver function tests have been seen in patients with NiV encephalitis21 and CSF chemistry resembles other non-haemorrhagic viral CNS infections.3
Neuroradiological features of NiV have been described and can help diagnose exposed individuals. Magnetic resonance imaging (MRI) studies have shown that extensive cortical involvement, particularly in the temporal lobe and pons, is common, while diffusion weighting imaging (DWI) illustrates multiple bilateral abnormalities (Fig 3).22 These changes are distinct from the characteristic features seen in HSV and JEV and are consistent with vasculitis-associated microangiopathy caused by NiV. Different MRI findings are seen in patients with late-onset and relapses of encephalitis, with their neuroimaging illustrating confluent hyperintense cortical lesions likely as a result of long standing micro-embolic damage from vasculitis.23
(a) MRI FLAIR imaging illustrating multiple small hyperintensities with cortical involvement. (b) MRI DWI illustrating multiple bilateral hyperintensities. Reproduced with permission from Anam et al.23
Management and prevention strategies
There are no licenced treatments or vaccines available for use in NiV to date. Treatment is limited to supportive care, which can include anti-seizure medications if seizures occur, treatment of secondary infections and mechanical ventilation in the case of respiratory failure.
Given the lack of definitive management options, prevention and containment of NiV outbreaks is crucial. Central to this is ensuring clinicians have a high index of suspicion for NiV cases and subsequently undertake detailed contact tracing and quarantining of possibly infected individuals. All those in contact with suspect NiV cases should adhere to wearing personal protective equipment with aerosol filtering masks when managing NiV patients given its propensity for nosocomial and household spread through droplet transmission.17
Provision of education around NiV spread is key to reducing transmission. This includes advising travellers to endemic regions to avoid consumption of fresh date palm sap or foraged foods and to be wary of close contact with fruit bats. In endemic regions, the use of barrier skirts around trees to prevent access by bats is advised. Finally, raising awareness among clinicians about the signs, symptoms and risk factors for NiV is necessary to ensure this pathogen with pandemic-potential is not forgotten.
Outcomes and neurological sequalae
Studies have illustrated the significant mortality and morbidity of NiV encephalitis – illustrating the need for early recognition to increase chances of survival and recovery. NiV has strikingly high case fatality with case studies reporting mortality ranging from 40 to 91% (Fig 4).18 A substantial number of patients surviving NiV suffer from persistent neurological and cognitive dysfunction including depression and deficits in memory. Disabling fatigue and functional impairment is also frequent of survivors of the disease.18,24
Bubble plot illustrating mortality rates throughout the Nipah virus outbreaks from 1999 to 2021.
Conclusion
The most recent outbreak of NiV in the end of 2021 in India illustrates the ongoing threat of this potentially pandemic-causing pathogen. It is a deadly zoonotic disease which can present non-specifically and causes fatal encephalitis. To date it has no approved therapeutic or vaccination options and resultantly surveillance and preventative measures remain key in controlling outbreaks. Clinicians should have a high index of suspicion in returning travellers from endemic regions presenting with signs and symptoms suggestive of encephalitis.
- © Royal College of Physicians 2022. All rights reserved.
References
- ↵
- ↵
- ↵
- McEntire CRS
- ↵UK Health Security Agency. High consequence infectious diseases (HCID). UKHSA, 2018. Available from www.gov.uk/guidance/high-consequence-infectious-diseases-hcid [Accessed 17 June 2022].
- ↵
- Johnson K
- ↵
- ↵
- Chandni R
- ↵
- Thomas B
- ↵
- ↵
- ↵
- ↵
- ↵
- Yadav PD
- ↵Soman Pillai V, Krishna G, Valiya Veettil M. Nipah virus: past outbreaks and future containment. Viruses 2020;12.
- ↵
- Sazzad HM
- ↵
- ↵
- Kenmoe S
- ↵
- ↵
- ↵Public Health England. Rare and Imported Pathogens Laboratory (RIPL) PHE Microbiology Services Porton Specimen Referral Guidelines and Service User Manual. PHE, 2014. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/367149/RIPL_user_manual_PHE_Version.pdf [Accessed 17 June 2022].
- ↵
- Lim CC
- ↵
- Anam AM
- ↵
Article Tools
Citation Manager Formats
Jump to section
Related Articles
- No related articles found.