Tracking cancer patients during COVID-19 using structured outcomes and keywords

ABSTRACT
For the duration of COVID-19, cancer pathways will be affected by the significant loss of elective capacity and increased risk of COVID-19-related morbidity and mortality for cancer patients. Imperial College Healthcare NHS Trust (ICHT) has developed a simple, effective MDT recording process, using keywords, to support the tracking of patients who require treatment prioritisation, repeated clinical/MDT reviews and/or need adjustments to their treatment. Following implementation in April, the percentage of MDT outcomes with keywords recorded was 79% in June and 77% for the first two weeks of July. Analysis of the 3,680 MDT outcomes with at least one key word recorded showed that 96% had the ‘intention to proceed’ recorded. For 59% patients, the decision was to ‘proceed’, 5% patients are being monitored, 3% patients have been deferred and 29% were ‘closed’. While this process adds time to busy MDTs, we hypothesise that it will support the tracking and safety-netting of thousands of cancer patients whose care has been affected by the pandemic. The process could easily be implemented in other trusts and adapted for other specialties.
Problem
MDT working is the gold standard for cancer patient management in the UK1 and many other healthcare systems, and the MDT meeting outcome is a critical part of the patient record. Good data collection, both for the benefit of the individual patient and for the purposes of audit and research, is one of the core principles of effective MDT working.2 MDT outcomes are recorded in different ways at different trusts. Imperial College Healthcare NHS Trust (ICHT) uses the Somerset Cancer Record (SCR), a software application developed by the NHS to support the collection of relevant data throughout a patient’s cancer journey. The application is used by over 95 organisations in England.3
For the duration of the COVID-19 outbreak, cancer pathways will be affected by the significant loss of elective capacity and patients undergoing active cancer treatment will be at increased risk of COVID-19-related morbidity and mortality due to deferred diagnostics and/or treatment, the adjustment of treatment plans in response to COVID-19 risk, and/or a COVID-19 diagnosis while on active cancer treatment. All trusts managing cancer patients will have made significant adjustments to their cancer pathways to respond to the new capacity pressures and to mitigate the risk of harm wherever possible. It is important that any adjustments to the diagnostic/treatment pathway are recorded clearly for each patient so that each member of the clinical team and the patient understands what adjustments were made, and why, and decisions can be audited (for example, in the event of a clinical incident). In time, the outcome data for patients whose pathways were adjusted due to COVID-19 will also be useful for research purposes – and to inform future changes to guidance during this pandemic, or any subsequent pandemics.
This paper outlines a potential solution for recording and tracking adjusted pathways. It also describes how this solution was developed and implemented, and the early results.
Potential solution
While there is a wealth of guidance on MDT meeting best practice,1,2,4,5 this has not yet been updated to reflect the information that needs to be recorded during COVID-19. ICHT has responded by developing a simple and effective MDT recording process to support the tracking, in the Somerset Cancer Register, of:
patients who need to be prioritised for any available elective capacity
patients who require repeated clinical/MDT reviews where treatment is not immediately possible
treatment plans adjusted in response to COVID-19 risk;
surgical patients eligible for referral to the Cancer Hub Clinical Prioritisation Group (CPG).
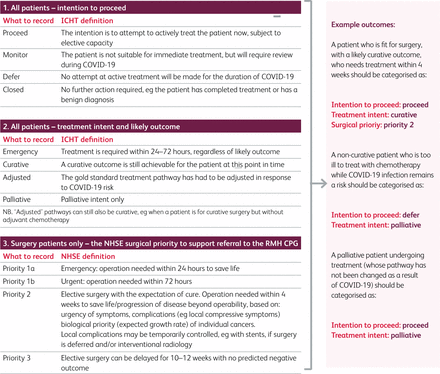
MDT leads must ensure that MDT outcomes are structured, including the specified information outlined in Box 1, and contain keywords to support patient categorisation and report generation (see Fig 1).
Information required for a structured MDT outcome
Outcome keywords. Each structured outcome needs to include one key word from section 1 and one from section 2 (and one from section 3 if the patient is having surgery). ICHT = Imperial College Healthcare NHS Trust; NHSE = NHS England; RMP CPG = Royal Marsden Partners Clinical Prioritisation Group.
The concept of structured outcomes is not new and will be familiar to clinicians. The incorporation of keywords was, however, a response to the need to track patients on adjusted pathways during COVID-19.
The development of the keywords was clinically led, drawing on the wealth of evidence around the quality and safety benefits of comprehensive and accurately coded data,6 and involved widespread consultation with ICHT’s MDT leads (surgeons and oncologists) and MDT coordinators. The keywords were developed and then trialled in a subset of MDTs. MDT leads and coordinators were asked to add the relevant keywords to the outcome in the ‘MDT comments’ box on the SCR. They were encouraged to do this during the meeting so that all MDT members could see and comment on what was being recorded. Changes, such as the addition of the ‘closed’ key word, were incorporated in response to specific feedback. The process was then rolled out to all MDTs through a combination of group introductory sessions with MDT leads and coordinators and one-to-one follow-up sessions. Any outcomes that were not coded during the initial implementation period were retrospectively coded by the Corporate Cancer Team and sent to the MDT leads for checking. Utilisation was tracked and reported on at the MDT leads meeting, a monthly or bimonthly meeting chaired by the divisional director of surgery, cardiovascular and cancer, where performance and service changes are discussed.
Results
Use of the keywords increased rapidly: following implementation of the process in April (when no keywords were recorded in this way), the percentage of MDT outcomes with keywords recorded was 79% in June and 77% for the first two weeks of July (time of writing). The remainder of management decisions were recorded as outcomes, but without the keywords to support tracking and safety-netting.
Inevitably, there were some MDTs who implemented the process very quickly (for example, prostate, haematology, lung) and others who took longer to adjust. We found that the MDTs who implemented the process quickly had several factors in common: they tended to be those where well-structured outcomes were already being used and there was a strong relationship between the MDT lead and the MDT coordinator.
The main challenge to implementation was scheduling time with the MDT leads and coordinators to explain the process and the anticipated benefits, during a time when staff were busy responding to the pressures caused by COVID-19. Implementation was also complicated by the simultaneous move to virtual MDT meetings and the teething problems associated with that, such as issues with microphone feedback and internet connectivity.
Analysis of the 3,680 MDT outcomes with at least one key word recorded showed that 96% had the ‘intention to proceed’ recorded. For 59% of patients, the decision was to ‘proceed’, 5% of patients are being monitored, 3% patients have had their treatment deferred and 29% were ‘closed’ (eg due to a benign diagnosis or completion of treatment). Of the 1,670 with a ‘treatment intent and likely outcome’ recorded, 5% patients were recorded as ‘emergency’, 13% as ‘adjusted’, 53% as ‘curative’ and 29% as ‘palliative’.
It is important to note that there is clearly a significant number of patients who are yet to enter the MDT system – 2 week wait referrals at ICHT are still only at 68% forecasted levels (weeks commencing 6 and 13 July). This follows a period of referrals dropping to as low as 24% forecasted levels (week commencing 6 April). The use of keywords does not address this problem, which is being targeted in other ways. For example, the ICHT cancer dashboard now includes referral data by borough (as well as by tumour site). This allows boroughs with lower than expected referral rates to be identified and supported. In addition, the Macmillan GP for Hammersmith and Fulham CCG now attends the MDT leads meeting to share insights from primary care to support discussions around recovery of referral activity.
Initial qualitative feedback from the clinical and management teams has been positive – and a more rigorous assessment of the process is underway. Examples of feedback received so far include:
‘At this difficult time for both patients and doctors this process was helpful, supportive and informative.’ (MDT clinical lead for breast cancer)
‘The process is really helping us to safety net our patients at a time when there have been significant changes to many of the clinical pathways. It has been easy to roll out and is supported by the clinical teams.’ (Senior member of the Corporate Cancer Team)
Reports are now being generated to show which patients have been deferred or are being monitored, and which patients are on an adjusted pathway. These were refined over several weeks to address issues with data entry (relating for example to spelling mistakes and typographical errors). The reports are shared with the MDT leads on a weekly basis so that they can keep track of these patients and restart the patient’s cancer pathway when appropriate and when elective capacity allows. Reports can be further refined at tumour level with additional data (eg stage, performance status, demographics) to support the management of a potentially large list of patients waiting for treatment or being monitored for disease progression. The reports are also being used to inform procurement decisions about independent sector capacity.
Conclusions and potential future
This process has given the clinical teams, and the Corporate Cancer Team, improved oversight of patients under ICHT’s care. While it was developed to support the tracking and safety-netting of patients during the pandemic, we expect that the concept of using keywords in MDT outcomes will continue to be useful post-COVID – for example in monitoring compliance with protocolised pathways. The approach could also be extended to other specialties.
The process was straightforward to implement and use of the keywords increased rapidly. This was felt to be down to a combination of careful framing of the process as a way of supporting better safety-netting and oversight of patients during the unprecedented challenges of the pandemic, strong clinical leadership, and close working with business intelligence colleagues on the development and design of the reports.
The process is only useful if clinicians and the Corporate Cancer Team use the reports to actively safety-net patients – for example by ensuring no patient whose management is deferred is ‘lost’ and all are scheduled for re-review when capacity increases or the risks relating to COVID-19 subside. Further work is needed to fully embed them into ICHT’s existing patient management and governance structures.
The process does also add time to already busy MDTs. Department of Health data shows a 20% year-on-year increase in the number of patient discussions had within an MDT meeting between 2011 and 2014/15.1 The impact of the additional time required to record keywords will need to be monitored as referrals return to normal levels and the number of patients listed for each MDT meeting increases again. However, our hypothesis is that better structuring of MDT outcomes and better coding of decisions will ultimately lead to fewer repeat discussions and more time available for the discussion of each patient. This hypothesis will need to be tested in due course.
ICHT will continue to iterate the approach in the face of subsequent waves of infection. Ideas for future changes include:
Moving to a drop-down menu data entry approach rather than a free-text approach. This will increase the accuracy of data entry and reporting.
Iteration of the elements of the structured outcome, potentially to include further information (pending consultation with the MDT leads, coordinators and other stakeholders) such as ‘treatment location’ (so that it is clear whether the patient is being treated within the Trust or at another trust), ‘person responsible for informing patient about plan’, and ‘indications for re-discussion (if any)’.
Further refinement of the reports to ensure they are a useful tool for the MDT leads and coordinators – for example by highlighting patients who were coded as ‘proceed’ but are yet to have received treatment.
- © Royal College of Physicians 2021. All rights reserved.
References
- ↵
- Gray R
- ↵
- National Cancer Action Team
- ↵
- Somerset NHS Foundation Trust
- ↵
- NHS England and NHS Improvement
- ↵
- Royal College of Radiologists
- ↵
- Swinglehurst D
Article Tools
Citation Manager Formats
Jump to section
Related Articles
- No related articles found.
Cited By...
- No citing articles found.