Abstract
Purpose
The relationship between the number of patients admitted to an intensive care unit (ICU) volume and mortality is currently the subject of debate. After implementation of a national guideline in 2006, all Dutch ICUs have been classified into three levels based on ICU size, patient volume, ventilation days, and staffing. The goal of this study is to investigate the association between ICU level and mortality of ICU patients in the Netherlands.
Methods
We analyzed data from 132,159 patients admitted to 87 ICUs between January 1, 2009 and October 1, 2011. Logistic GEE analyses were performed to assess the influence of ICU level on in-hospital mortality and 90-day mortality in the total ICU population and in different ICU subgroups while adjusting for severity of illness by APACHE IV.
Results
No significant differences were found in the adjusted in-hospital mortality of the total ICU population and in different subgroups admitted to level 1, 2 and 3 ICUs. In-hospital mortality in level 2 and 3 ICUs as opposed to level 1 ICUs was 1.06 (0.93–1.22) and 1.10 (0.94–1.29), respectively, and 90-day mortality was 0.92 (0.80–1.06) and 1.01 (0.88–1.17).
Conclusion
We demonstrated that ICU level was not associated with significant differences in the case-mix adjusted in-hospital and long-term mortality of ICU patients. This finding is in contrast with some earlier studies suggesting a volume–outcome relationship. Our results may be explained by the successful implementation of nationwide mandatory quality requirements and adequate staffing in all three levels of ICUs over the last years.
Similar content being viewed by others
Background
Numerous studies have focused on the relationship between volume of patients admitted to intensive care units (ICUs) and outcomes. Recent evidence favors higher volume hospitals for complex planned surgical procedures. Such a volume–outcome relationship also exists for some medical conditions such as diabetes, myocardial infarction and post-cardiac arrest. A policy of centralization of services, especially for certain surgical procedures, has evolved on this basis [1].
Kahn et al. [2], utilized risk estimates to project that 4,720 lives per year (95 % range 2,522–6,744) could potentially be saved in eight states of the USA by routinely transferring ICU patients from low- to high-volume hospitals. However, it is important to note that several local organizational factors, such as ICU training, staffing, closed/open format, admission policy, case-mix, transfer protocols, etc., may have a significant impact on outcome in ICUs. Consequently, the results of this study cannot be extrapolated directly to other country settings. Kanhere et al. [1] found that some types of patients may fare better in high-volume ICUs. Their review included mostly retrospective studies, which were prone to bias. There were inconsistencies in the study results after adjusting for patient risk, and, in a recent structured abstract, the results of this review were therefore found unlikely to be reliable [3]. Some studies conducted on ICU patients have shown an advantage for (subgroups of) patients treated in high-volume centers [4–6]. An earlier Dutch study with data from 2003 to 2005 found a volume–outcome relationship for septic patients but included only five low-volume ICUs [7]. Other studies were either unable to demonstrate a volume effect [8, 9] or found it only in subgroups (i.e. patients with gastrointestinal diagnoses [10] and patient subgroups depending on other procedural factors such as volume of surgery [11]). A study in Australia and New Zealand showed an incremental increase in mortality with patient volume [12]. A recent review [13] found that patient mortality may be improved in large capacity ICUs. However, a lack of consistent findings in different patient types suggested other factors needed to be considered in addition to volume, in particular structural characteristics of the organization such as staffing models and case-mix.
The 2006 Dutch Intensive Care guideline [14] defined three levels of ICU care based on annual patient volume, number of ICU beds, number of ventilation days, and physician and nurse staffing (see supplementary material Appendix 1). The guideline includes an established quality system for all levels of ICU: the implementation of this guideline in the Netherlands was completed by December 11, 2008 in 89 % of Dutch ICUs [15] and by July 15, 2009 in all Dutch ICUs [16].
The purpose of this study is to evaluate the relationship between ICU level and the case-mix adjusted in-hospital mortality in the Netherlands. To adjust for differences in referral policy, we also evaluated the relationship between ICU level and the case-mix adjusted 90-day mortality of Dutch ICU patients.
Methods
Data
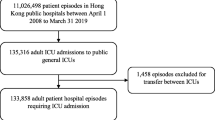
We conducted a cohort study on ICU patients admitted between January 1, 2009 and October 1, 2011. Data were extracted from the Dutch National Intensive Care Evaluation (NICE) registry [17]. The NICE registry started in 1996 and includes demographic, physiological and clinical data of all ICU patients admitted to participating ICUs, including, but not limited to, primary diagnosis at admission, age, location of the patient before ICU admission, length of hospital stay before ICU admission, ventilator status, ICU and in-hospital mortality, and the Acute Physiology and Chronic Health Evaluation (APACHE) IV variables [18]. In our study, we used the predicted mortality risk of patients according to the APACHE IV model to correct for case-mix differences. Therefore, we could only include the ICU admissions that fulfilled the inclusion criteria of the APACHE IV model [18]. Furthermore, we excluded cardiac surgical patients. In Fig. 1, a flowchart of the inclusion of ICU patients is given.
During the study period, 87 Dutch mixed medical–surgical ICUs, more than 90 % of all Dutch ICUs, participated in the NICE registry. All participating centers are obliged to attend data collection training sessions to ensure consistency and quality of the NICE data, according to the stated data definitions reported in the NICE data dictionary. The data are checked for range and consistency by computer algorithms. Also, an onsite data quality audit is conducted periodically to ensure the validity of the data [19].
The registration of organizational information is additional to the patient information registry within the NICE. This organizational information can be provided voluntarily to the NICE registry. Information on number of beds and staff is provided by 78 ICUs (39 level 1 ICUs, 25 level 2 ICUs, and 14 level 3 ICUs). Information on the presence and availability of intensivist is provided by 67 ICUs (31 level 1 ICUs, 24 level 2 ICUs, and 12 level 3 ICUs). Information on bed occupancy and IC nurse/patient ratio is provided by 63 ICUs (25 level 1 ICUs, 25 level 2 ICUs, and 13 level 3 ICUs).
To minimize the possible effect of lower in-hospital mortality due to referral of sicker patients to centers with a higher ICU level (referral bias), we also determined mortality 90 days after ICU admission. The NICE registry includes data until hospital discharge. Therefore, the long-term mortality was assessed by linking the NICE registry to the insurance claims database of Vektis [20]. Vektis is a center for information and standardization of health data, initiated by the Dutch health insurance companies. It collects and analyses data regarding costs and quality of the Dutch health system and is maintained by the Dutch Ministry of Health. A deterministic linkage algorithm [21] using gender, date of birth, ICU admission date, and ICU discharge date of each admitting hospital was conducted. In this method, the variables in both databases must be identical for a positive match. By this procedure, the vital status (death or alive) on January 1, 2012, and if relevant, the date of death was added to the NICE registry. To avoid bias due to incomplete linkage, only ICUs of which the data could be linked for more than 75 % of the ICU admissions were included. The final dataset used in this study has been encrypted, removing all patient identifying information. Anonymized data use does not require informed consent in the Netherlands, and data were officially registered in accordance with the Dutch Personal Data Protection Act [22].
Definition of ICU levels
Included ICUs were classified into three levels as defined by the 2006 national Intensive Care guideline [14]. All ICUs are mixed medical–surgical units organized in a closed format system with intensivists who coordinate care together with the admitting specialist. A level 1 ICU is defined as an ICU with a minimum of 6 beds, up to 1,249 ventilation days/year and at least 2 intensivists who coordinate patient care. A level 2 ICU is defined as an ICU with a minimum of 12 beds, 1,250–1,499 ventilation days, at least 0.35 full time equivalent (FTE) intensivists and 0.45 FTE house doctors per ICU bed (based on the actual number of staff employed). A level 3 ICU is defined as an ICU with a minimum of 12 beds, more than 1,500 ventilation days and at least 0.45 FTE intensivists and 0.55 FTE house doctors per ICU bed (see supplementary material Appendix 1). All levels ICU had to have a minimum nurse/patient ratio of at least 1:2 (level 1 ICU), 1:1, 5 (level 2 ICU) or 1:1, 3 (level 3 ICU) actually being on duty. Implementation of a mandatory selection of ICU protocols as stated by the Dutch Society of Intensive Care (i.e. each ICU had to have such a protocol but it could be adapted to the hospital situation and hospital format), and other quality instruments such as a daily multidisciplinary patient conference, regular complication and necrology conferences, collection of a minimal set of quality parameters and an annual report, were mandatory for all ICU levels. For patients expected to be ventilated in excess of 72 h, a level 1 ICU had to consult with (but not to transfer to) a higher level ICU within their geographical region (regionalization). The guideline encourages regionalization of the Dutch ICUs, but it is the only item not yet fully implemented by 2014 and was thus not implemented in the study period either. The three levels of ICU care studied, comprise differences in patient volume, case-mix, ICU size, annualized ventilation days and staffing, whereas earlier studies used only patient volume as a variable. It is therefore possible that there are level 1 ICUs with a larger patient volume than a given level 2 ICU but with fewer annualized ventilation days, smaller ICU size or less staffing. The same applies for level 2 and 3 ICUs.
Statistical analysis
In order to analyze the relationship between ICU level and mortality, we used logistic generalized estimating equation (GEE) models, which account for potential correlation of outcomes within ICUs [23]. The GEE models were used to calculate the odds ratios and 95 % confidence intervals (CI) of ICU level while adjusting for the severity of illness of the patients. While applying the GEE models, the APACHE IV model was first level recalibrated as we used the logit-transformed original APACHE IV mortality risk to adjust for the severity of illness during the analysis. The relationship between ICU level and mortality was analyzed for the ICU population as a whole, as well as for ICU subgroups based on admission type (e.g., medical, elective surgical, emergency surgical), APACHE IV predicted mortality risk (e.g., 30–50, 50–70, >70 %), ventilation in the first 24 h of ICU admission, and reason for ICU admission (e.g., sepsis, pneumonia, intoxication, primary airway problems, out of hospital cardiac arrest (OHCA), colorectal surgery) (for definitions, see supplementary material Appendix 2). For each subgroup, we used two logistic GEE models, one for the outcome variable in-hospital mortality and one for the outcome variable mortality 90 days after ICU admission. We compared population characteristics across three ICU levels using a Mann–Whitney U test for non-normally distributed continuous variables and the χ 2 test for categorical variables.
All statistical analyses were performed using PASW® statistics 18 (SPSS, Chicago, IL, USA) and the R environment for statistical computing version 2.15.1 [24].
Results
A total of 191,264 ICU patients were admitted to 87 mixed medical-surgical ICUs between January 1, 2009 and October 1, 2011. In the analysis of in-hospital mortality, 132,159 ICU patients were included, and in the analysis of mortality 90 days after ICU admission, 115,909 patients (93.6 %) from 83 ICUs (40 level 1 ICUs, 27 level 2 ICUs, and 16 level 3 ICUs) were included. A flowchart of patient entry into the study is shown in Fig. 1.
Characteristics of the different ICUs and patients are shown in Table 1.
The severity of illness expressed as the APACHE IV score was lower in level 1 ICUs than in level 2 and 3 ICUs; the median APACHE IV score being respectively 49 versus 54 versus 54. Level 3 ICUs admitted more patients from other hospitals (4.9 %) compared to level 2 (2.6 %) and level 1 (1.9 %) ICUs.
Table 2 shows the organizational characteristics of the three ICU levels.
Table 2 shows that level 3 ICUs did have more hospital beds. The bed occupancy in level 3 ICUs is higher than in level 1 and 2 ICUs and the ratio IC nurse/patient (e.g., number of FTE IC nurses actually on duty divided by the number of IC patients present) in level 3 ICUs is lower than in level 1 ICUs.
In Table 3, the odds ratios for hospital mortality and 90-day mortality of level 2 and 3 ICUs for the total IC population and for each subgroup are given, with the level 1 ICUs as reference, to determine whether there are significant differences between level ICU and in-hospital mortality and 90-day mortality.
The odds ratios mostly showed non-significant differences between in-hospital mortality and 90-day mortality and the level of ICU. In one subgroup, the odds ratio for in-hospital mortality differed significantly: in-hospital mortality for ventilated patients in level 3 ICUs was higher than in level 1 ICUs. The odds ratio based on 90-day mortality showed no significant differences for the ICU population as a whole or any subgroup.
Discussion
This study suggests that there was no association between ICU level of care and case-mix adjusted in-hospital or long-term mortality in Dutch ICU patients. This was also true for several subgroups based on admission type, reason for ICU admission, or predicted APACHE IV mortality. Only in the subgroup of ventilated patients was the in-hospital mortality in level 3 ICUs higher than in level 1 ICUs. This difference was not present in 90-day mortality. Neither of the subgroups of patients that do not necessarily need to be transferred to higher level ICUs and high risk patients showed a significant difference in mortality between level 1, level 2, and level 3 ICUs. The absence of a difference in mortality in all patient groups supports our hypothesis that the level of ICU does not influence outcome.
Low level ICUs had a higher nurse/patient ratio than higher level ICUs. This is probably explained by the fact that the high level ICUs had more ICU nurses in training (who are not accounted for in the NICE registration).
Contrary to other studies looking at patient volume and outcome [4–12], our study was carried out on three different levels of ICU classified by patient volume, ventilation volume, nurse and physician staffing. To our knowledge, this is the first study focusing on ICU level and outcome and not merely on patient volume–outcome relationship. Kahn et al. [4] examined data of 20.241 ventilated medical patients in the USA and found a lower ICU mortality in high-volume hospitals. However, we showed that the adjusted in-hospital mortality of mechanically ventilated patients in level 3 ICUs was higher than in level 1 ICUs. The fact that we did not find a relationship between level of care and mortality may be caused by implementing the 2006 Dutch Intensive Care guideline, which states that every Dutch ICU has a minimum size of at least six beds, a nurse/patient ratio of at least 1:2 and at least two intensivists working on the ICU including a formal head of department. In 2008, when 89 % [24] of the Dutch ICUs had successfully implemented the 2006 ICU guideline [14], the Dutch health authorities encouraged and accelerated the implementation process, resulting in the fact that, by July 15, 2009, all guideline requirements except regionalization were fully implemented in all Dutch ICUs [16]. In contrast to the Dutch situation, the implementation process of ICU quality criteria in the USA showed that, by 2000, only 4 % of all the ICUs had implemented the Leapfrog criteria [26], and by 2010 it was only 34 % [27], whereas in our study, a similar quality implementation process was completed in all the participating ICUs [16]. This may explain the positive correlation between ICU volume and outcome in the USA studies which is not found in our study. The only study showing mortality increments with patient volume [12] is from Australia and New Zealand, with a similar ICU system as in the Netherlands: unique features are organization, training and relationship of nursing and medical staff, the model of training and operation of ICU, the role of physiotherapy in patient care, and the independence of the intensive care department from other specialties [28], all very similar to the Dutch system and supporting our suggestion that a homogeneous implementation of a stringent quality system leads to good ICU outcome on all levels of ICU. A recent Spanish study with closed-format ICU models and 24/7 coverage by trained ICU physicians also showed no volume–outcome relationship [9]. Caution should be applied when comparing results from one country or health-care system to another, as international differences in critical care unit provision, structure and organization do exist and may limit the generalizability of these findings [29]. We have analyzed the Dutch situation with a unique system of three different levels of ICUs; other specific features of the Dutch ICU system are a low threshold for ICU admission and an overall model of mixed ICUs. Furthermore, differences between European countries also exist, as the Netherlands has lower mortality rates in comparison to the UK (ICNARC) and France. We thus cannot guarantee that our results are generalizable to other countries.
During our analysis, we adjusted for several case-mix differences by using the APACHE IV mortality risks, and we adjusted for the fact that patients within a hospital are more alike than patients between different hospitals by using a GEE model. However, we cannot rule out that there are case-mix factors influencing the outcome of patients for which the APACHE IV does not or insufficiently adjust. In a former study of Brinkman et al. [25], for example, it is illustrated that hospitals that admit more severely ill patients are disadvantaged compared to hospitals admitting less severely ill patients when calculating the SMR. Furthermore, we analyzed the case-mix adjusted mortality of level 1, 2, and 3 ICUs of the ICU population actually admitted to these ICU levels. It is unknown how the case-mix adjusted mortality would be if, for example, the ICU population now admitted to level 3 ICUs were admitted to level 1 ICUs or vice versa. Patients transferred to an ICU of another level were included in the SMR of the referring ICU, which may introduce a referral bias. Therefore, we also calculated the odds ratio ICUs level 2 and 3 ICUs with level 1 ICUs as reference, excluding the patients transferred to another hospital (4, 2 % of the ICU population). These additional results shown in supplementary material Appendix 4 are similar to the odds ratios including the transferred patients (Table 3). Furthermore, by calculating the 90-day mortality, we adjusted for any referral bias. Although in most subgroups not significant, the in-hospital mortality of level 1 ICUs seems to be lower compared to level 2 and 3 ICUs, while it is the opposite for 90-day mortality. The reason for the trend towards lower in-hospital mortality in level 1 ICUs may be explained by the fact that level 1 ICUs transfer more patients, which thus automatically survive the hospitalization in the transferring level 1 ICU. In cases where the patient dies in the higher level ICU, this death will only be counted towards the transferring level 1 ICU if the mortality 90 days after ICU admission is calculated. Another example is when patients are transferred to a hospice which results in a lower in-hospital but a higher 90-day mortality.
The strength of this study is its very large sample size including almost all of the Dutch national ICUs and the analysis of relevant subgroups.
The absence of an ICU level–outcome relationship, as illustrated by the fact that after exclusion of all transferred patients no significant differences in in-hospital mortality remained (see supplementary material Appendix 4), suggests that referral from a low to a medium or high level ICU or vice versa should be based on the presence or absence of more specialized diagnostic and treatment facilities in these hospitals, and not on the premise of better provision of care or outcome in higher or lower level ICUs as such.
More information is needed about the structure, process, and organization of care within level 1, 2 and 3 ICUs to identify the best way to improve outcome. Future research should focus on physician case volume as opposed to ICU level or hospital volume, and the role of regionalization in IC treatment (mandatory consulting and referral of specific patient groups).
Conclusion
Our findings suggest that all ICUs—low, medium and high level—provide similar outcomes for the ICU patients admitted to these ICUs. Implementation of mandatory quality requirements, such as minimal staffing, adherence to national IC protocols, a daily multidisciplinary patient conference, regular complication and necrology conferences, collection of a minimal dataset of quality parameters and an annual report, is in our opinion the key factor for the comparable outcome in all three ICU levels.
References
Kanhere MH, Kanhere HA, Cameron A, Maddern GJ (2012) Does patient volume affect clinical outcomes in adult intensive care units? Intensive Care Med 38:741–751
Kahn JM, Linde-Zwirble WT, Wunsch H et al (2008) Potential value of regionalized intensive care for mechanically ventilated medical patients. Am J Respir Crit Care Med 177:285–291
Centre for Reviews and Dissemination (2014) Does patient volume affect clinical outcomes in adult intensive care units? (Structured abstract). Database of Abstracts of Reviews of Effects 2014; Issue 3. Available online at http://onlinelibrary.wiley.com/o/cochrane/cldare/articles/DARE-12012030834/frame.html. Accessed 18 Oct 2014
Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD (2006) Hospital volume and the outcomes of mechanical ventilation. New Engl J Med 355:41–50
Shahin J, Harrison DA, Rowan KM (2014) Is the volume of mechanically ventilated admissions to UK critical care units associated with improved outcomes? Intensive Care Med 40:353–360
Walkey AJ, Wiener RS (2014) Hospital case volume and outcomes among patients hospitalized with severe sepsis. Am J Respir Crit Care Med 189:548–555
Peelen L, de Keizer NF, Peek N, Scheffer GJ, van der Voort PHJ, de Jonge E (2007) The influence of volume and intensive care unit organisation on hospital mortality in patients admitted with severe sepsis: a retrospective multicentre cohort study. Crit Care 11:R40
Cooke CR, Kennedy EH, Wiitala W, Almenoff P, Sales AE, Iwashyna TJ (2012) Despite variation in volume, Veterans Affairs hospitals show consistent outcomes among patients with non-postoperative mechanical ventilation. Crit Care Med 40:2569–2575
Fernandez R, Altaba S, Cabre L et al (2013) Relationship between volume and survival in closed intensive care units is weak and apparent only in mechanically ventilated patients. Anesthesiology 119:871–879
Durairaj L, Torner JC, Chriskhilles EA et al (2005) Hospital volume-outcome relationships among medical admissions to ICUs. Chest 128:1682–1689
Reinikainen M, Karlsson S, Varpula T et al (2010) Are small hospitals with small intensive care units able to treat patients with severe sepsis? Intensive Care Med 36:673–679
Moran JL, Solomon PJ, ANZICS Centre for Outcome and Resource Evaluation of the Australian and New Zealand Intensive Care Society (2012) Mortality and intensive care volume in ventilated patients from 1995 to 2009 in the Australian and New Zealand binational adult patient intensive care database. Crit Care Med 40:800–812
Abbenbroek B, Duffield CM, Elliott D (2014) The intensive care unit volume–mortality relationship, is bigger better? An integrative literature review. Aust Crit Care 27:157–164. doi:10.1016/j.aucc.2014.02.001 (Epub 2014 Mar 11)
Dutch National Intensive Care Guideline (2006) Available online at http://nvic.nl/sites/default/files/Richtlijnen%20aanmaken/Intensive%20Care%202006%2C%20CBO%20Richtlijn.pdf. Accessed 12 Apr 2014
IC-afdelingen van niveau 1: “op weg naar verantwoorde zorg” Available online at http://www.igz.nl/onderwerpen/curatieve-gezondheidszorg/ziekenhuizen/intensive-care/. Accessed 14 Apr 2014
“Alle 50 ziekenhuizen met een Intensive Care (IC) op niveau 1 voldoen aan de voorwaarden voor verantwoorde zorg.” Available online at http://www.igz.nl/actueel/nieuws/alle50ziekenhuizenvoldoenaanic1normen.aspx?sgURI=tcm%3a294-47450-4&nodeJump=4. Accessed 13 Apr 2014
Dutch National Intensive Care Evaluation (NICE) foundation Available online at http://www.stichting-nice.nl. Accessed 13 Apr 2014
Zimmerman JE, Kramer AA, McNair DS, Malila FM (2006) Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 34:1297–1310
Arts D, de Keizer N, Scheffer GJ, De Jonge E (2002) Quality of data collected for severity of illness scores in the Dutch National Intensive Care Evaluation (NICE) registry. Intensive Care Med 28:656–659
Vektis Available online at http://www.vektis.nl. Accessed 14 Apr 2014
Roos LL, Wajda A (1991) Record linkage strategies. Part I: estimating information and evaluating approaches. Methods Inf Med 30:117–123
http://www.dutchdpa.nl/Pages/en_ind_cbp.aspx. Accessed Apr 14, 2014
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121–130
R Development Core Team (2011) R: A Language and Environment for Statistical Computing. The R Foundation for Statistical Computing, Vienna. Available online at http://www.R-project.org/ Accessed 18 Oct 2014
Brinkman S, Abu-Hanna A, van der Veen A, de Jonge E, de Keizer NF (2012) A comparison of the performance of a model based on administrative data and a model based on clinical data: effect of severity of illness on standardized mortality ratios of intensive care units. Crit Care Med 40:373–378
Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR Jr (2000) Improving the safety of health care: the Leapfrog initiative. Eff Clin Pract 3:313–316 (Erratum, Eff Clin Pract 2001;4:94)
Factsheet Leapfrog group (2010) Available online at www.leapfroggroup.org/media/file/FactSheet_IPS.pdf. Accessed 15 Apr 2014
Bellomo R, Stow PJ, Hart GK (2007) Why is there such a difference in outcome between Australian intensive care units and others? Curr Opin Anaesthesiol 20:100–105
Wunsch H, Angus DC, Harrison DA et al (2008) Variation in critical care services across North America and Western Europe. Crit Care Med 36:2787–2793
Acknowledgments
Johannes van der Hoeven is receiving grants for his institution for expert testimony, research and lectures. Crétien Jacobs and Giel van Berkel are honorary members of the “National Intensive Care Evaluation” foundation (NICE) and get paid their expenses from the “National Intensive Care Evaluation” foundation (NICE). Sylvia Brinkman and Nicolette F. de Keizer are working for the “National Intensive Care Evaluation” foundation (NICE). Yvonne E.M. Snel is working for the Association of General Hospitals (40 hospitals). The Association of General Hospitals has asked the “National Intensive Care Evaluation” foundation (NICE) to investigate the association between the 3 Dutch ICU levels and mortality of ICU patients.
Conflicts of interest
Georg H. Kluge, John P.W. Vogelaar and Emiel S. Boon declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Take-home message: Analysis of data from 132,159 patients admitted to 87 ICUs between 2009 and 2011 showed no significant differences in the adjusted in-hospital and 90-day mortality of the total ICU population and in different subgroups admitted to level 1 (small), 2 (medium-sized) and 3 (large) ICUs. Our results may be explained by the successful implementation of mandatory quality requirements and minimal staffing requirements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kluge, G.H., Brinkman, S., van Berkel, G. et al. The association between ICU level of care and mortality in the Netherlands. Intensive Care Med 41, 304–311 (2015). https://doi.org/10.1007/s00134-014-3620-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-014-3620-1