Abstract
ARDS, first described in 1967, is the commonest form of acute severe hypoxemic respiratory failure. Despite considerable advances in our knowledge regarding the pathophysiology of ARDS, insights into the biologic mechanisms of lung injury and repair, and advances in supportive care, particularly ventilatory management, there remains no effective pharmacological therapy for this syndrome. Hospital mortality at 40% remains unacceptably high underlining the need to continue to develop and test therapies for this devastating clinical condition. The purpose of the review is to critically appraise the current status of promising emerging pharmacological therapies for patients with ARDS and potential impact of these and other emerging therapies for COVID-19-induced ARDS. We focus on drugs that: (1) modulate the immune response, both via pleiotropic mechanisms and via specific pathway blockade effects, (2) modify epithelial and channel function, (3) target endothelial and vascular dysfunction, (4) have anticoagulant effects, and (5) enhance ARDS resolution. We also critically assess drugs that demonstrate potential in emerging reports from clinical studies in patients with COVID-19-induced ARDS. Several therapies show promise in earlier and later phase clinical testing, while a growing pipeline of therapies is in preclinical testing. The history of unsuccessful clinical trials of promising therapies underlines the challenges to successful translation. Given this, attention has been focused on the potential to identify biologically homogenous subtypes within ARDS, to enable us to target more specific therapies ‘precision medicines.’ It is hoped that the substantial number of studies globally investigating potential therapies for COVID-19 will lead to the rapid identification of effective therapies to reduce the mortality and morbidity of this devastating form of ARDS.
Similar content being viewed by others
Several ARDS therapies show promise in clinical studies, while a growing pipeline of therapies is in preclinical testing. The history of unsuccessful clinical trials of promising therapies underlines the challenges to successful translation. Attention is now focused on identifying biologically homogenous subtypes within ARDS, to enable us to identify more specific ‘precision medicines’ for this severe syndrome. |
Introduction
Acute respiratory distress syndrome (ARDS) is the commonest form of acute severe hypoxemic respiratory failure in the critically ill. First described in 1967, the management of ARDS remains supportive [1]. Despite considerable advances in our knowledge regarding the pathophysiology of ARDS, insights into the biologic mechanisms of injury and lung repair, and advances in supportive care, particularly ventilatory management, there remains no effective direct therapy for ARDS. Mortality and morbidity remain unacceptably high [2], underlining the need to continue to develop and test therapies for this devastating clinical condition. The lack of effective ARDS therapies has been further highlighted in the evolving COVID-19 pandemic, which causes severe acute respiratory failure and ARDS in 3–5% of infected patients. The prior disappointing experience with potentially promising therapies that have subsequently failed in large-scale clinical trials must also be borne in mind [3].
In this review, we assess the current status of promising emerging therapies for patients with ARDS. We focus on drugs that: (1) modulate the immune response, both via pleiotropic mechanisms and via specific pathway blockade effects, (2) modify epithelial and channel function, (3) target endothelial and vascular dysfunction, (4) have anticoagulant effects, and (5) enhance ARDS resolution. We also critically assess drugs that demonstrate potential in emerging reports from clinical studies in patients with COVID-19-induced ARDS.
Therapies in clinical trials for ARDS
Immunomodulatory therapies
A number of medications with a broad base of ‘pleiotropic’ immunomodulatory effects are in clinical trials for the treatment of ARDS or to prevent ARDS development (Figs. 1, 2).
Steroids
Steroids have long been studied as a potential therapy for both early and late phase ARDS, with some studies suggesting potential benefit, via suppression of the pro-inflammatory cytokine response, while other studies demonstrating potential risks due to immune suppression. A recent interesting open-label multicenter study examined the efficacy of high-dose dexamethasone regimen in patients with established moderate to severe ARDS (i.e., P/F ratio < 200 mmHg at 24 h following ARDS diagnosis). Although terminated early for low recruitment, it was found that the mean number of ventilator free days was 4.8 days higher and the number of patient deaths was lower (21% versus 36%) following early treatment with dexamethasone [4]. The authors highlight the dosing regimen and time of administration as key to the use of steroid therapy in ARDS. Additional studies, focused on this specific moderate to severe ARDS population (diagnosed within 24 h), will be required to confirm and extend these interesting findings.
Ulinastatin
Ulinastatin is a urinary glycoprotein and protease inhibitor with potent antioxidant and anti-inflammatory effects [5]. In a small phase 2 trial, patients (n = 40 per group) with ARDS treated with ulinastatin injection (12 hourly for 14 days) demonstrated improved lung oxygenation and function and reduced duration of mechanical ventilation and reduced hospital stays compared to standard care [5]. Ulinastatin therapy also significantly lowered inflammatory cytokines and increased antioxidant activities [5]. Another phase 2 trial of ulinastatin is currently enrolling, and a number of other protease inhibitors are in the preclinical stages of testing.
Vitamin C
Vitamin C is recognized for its antioxidant and reparative properties. In a phase 2 study of patients with sepsis-induced ARDS, vitamin C did not reduce SOFA scores, which was the primary outcome, nor did it have an effect on biomarkers, even at high doses [6]. Of the secondary outcomes, vitamin C did reduce 28-day mortality. The time delay between onset of shock and development of ARDS delayed the administration of Vitamin C infusion when compared to other studies in sepsis [6]. A phase 2 trial is currently recruiting SARS-CoV-2 patients for treatment with vitamin C (NCT04254533).
Carbon monoxide
Carbon monoxide (CO) is a gas produced endogenously by heme oxygenase, which protects against oxidative stress, cell death and suppresses inflammation [7]. Preclinical lung injury studies have shown safety and promising efficacy of low-dose inhaled CO [8]. In an exploratory phase 1 study, eight patients with ARDS were treated with inhaled low-dose CO (100–200 parts per million), which was well tolerated with trends toward a difference in lung injury severity score and a trend toward improved SOFA scores in the treatment group [9]. A phase 2 efficacy study of CO in ARDS is currently recruiting.
Mesenchymal stromal cell (MSC) therapies
MSCs have immunomodulatory and pro-reparative effects and show efficacy in preclinical models of ARDS [10, 11]. A single IV infusion of allogeneic, bone marrow-derived human MSCs was well tolerated in nine patients with moderate to severe ARDS in a 2015 phase 1 dose escalation trial [12]. However, in the subsequent phase 2a study in 60 participants, MSC treatment did not improve outcomes [13]. MSC viability was variable and may have altered their efficacy, while the patient group that had received MSC therapy was more severely ill at baseline [13]. A phase 1 study of an umbilical cord derived MSC in moderate–severe ARDS showed safety and potentially interesting immunomodulatory effects [14]. A preliminary report from an unpublished phase 1/2 trial of MultiStem® (bone-marrow-derived human MSCs) suggested that MultiStem® therapy enhanced the number of ventilator-free days (VFDs) and ICU-free days and lowered mortality [15]. Another MSC trial using umbilical cord derived cells is currently recruiting (NCT03042143), and two others are ongoing (NCT02444455, NCT03608592).
Pathway-specific immunomodulators to prevent ARDS
Dilmapimod
The p38 mitogen-activated protein kinase (MAPK) pathway is activated during cellular stress and drives downstream production of inflammatory cytokines [16]. Dilmapimod is a specific p38MAPK inhibitor and potent anti-inflammatory. In a small dose response study in trauma patients at risk for ARDS development, a 24-h dilmapimod infusion was well tolerated and reduced the concentrations of the pro-inflammatory cytokines IL-6, IL-8 and soluble tumor necrosis factor receptor 1 (TNFR1) [16]. The incidence of ARDS was low overall and not different between the groups [16].
Anti-TNFR1
An anti-TNFR1 antibody selectively antagonizes TNF-α signalling through TNF receptor-1 (TNFR1), but not through TNFR2. In a volunteer study in 37 healthy humans challenged with a low dose of inhaled LPS, anti-TNFR1 attenuated pulmonary neutrophil infiltration, inflammatory cytokine release, and reduced evidence of endothelial injury [17]. Targeting TNFR1 may have potential in ARDS and requires further investigation.
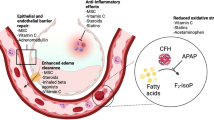
Therapies targeting epithelial/endothelial dysfunction
ARDS is a disorder involving injury and dysfunction of the pulmonary epithelium and endothelium, with resultant dysfunction of the alveolar–capillary barrier leading to lung edema. Consequently, targeting epithelial ion channels/channel dysfunction and endothelial/vascular dysfunction in ARDS constitute an important therapeutic target.
AP-301
AP-301 (also termed Solnatide) is an activator of alveolar epithelial sodium channels. Nebulized AP-301 every 12 h for 7 days was recently shown to decrease extravascular lung water and reduce ventilation pressures in a small phase 2 (n = 20 per group) randomized blinded exploratory study in patients with early ARDS (< 48 h of diagnosis) stratified based on SOFA score (SOFA score ≥ 11) [18]. Another, larger phase 2 study of AP-301 for the treatment of pulmonary edema in patients with moderate–severe ARDS is currently recruiting (NCT03567577), while another is recruiting COVID-19 ARDS patients (EudraCT Number: 2020-001244-26).
Citrulline
This nonessential amino acid is a substrate for nitric oxide synthase (NOS) in the formation of nitric oxide (NO). Low levels of citrulline are seen in patients with ARDS [19]. Citrulline deficiency may cause NOS to produce harmful nitrites, while a drop in NO can induce vasodilation, leukocyte adhesion, and alter other important aspects of endothelial function [19]. A recently completed, small phase 2 study of lower-dose (n = 26) versus higher-dose (n = 24) citrulline for patients with sepsis-induced ARDS showed no effect over placebo (n = 22) on the primary outcome measure (vasopressor dependency index), but a full report has not been published (NCT01474863).
ACE2
Angiotensin II is a vasoconstrictor, which has been implicated in lung inflammation and pulmonary edema, and is inactivated by angiotensin-converting enzyme 2 (ACE2). Angiotensin (1–7), the product of ACE2, attenuates ventilator- or acid aspiration-induced lung injury and inflammation [20] and reduces post-injury lung fibrosis [21]. Recombinant ACE2 administration was well tolerated in a phase 1 dose escalation study, while in the subsequent phase 2a study of 39 ARDS patients with concomitant infection/sepsis, there were no differences in lung or SOFA scores between the treatment and placebo groups [22].
Anticoagulants and thrombolytic therapies
Dysfunction of coagulation in ARDS plays a key role in ARDS pathogenesis. Consequently, anticoagulants and thrombolytics have also received attention as therapies for ARDS.
ALT-836
Tissue factor (TF) is a glycoprotein that is upregulated in the lung during inflammation and leads to fibrin deposition which incites further inflammatory effects [23]. Studies have observed that increased TF in the serum of ARDS patients correlates with higher mortality [23]. The anti-TF drug, ALT-836, was found to be safe when administered to ARDS patients in a phase 1, randomized, placebo-controlled, dose escalation study [24]. A phase 2 efficacy study of ALT-836 in 150 septic patients with ARDS was completed in 2013, but these results have not been published.
Heparin
Both heparin and antithrombin have been shown to dampen inflammation and ALI in preclinical models without negatively impacting systemic coagulation [25]. Nebulized heparin reduced the need for mechanical ventilation in a small phase 2 study of 50 critically ill patients [26]. Prophylactic nebulized heparin enhanced alveolar perfusion and CO2 elimination in patients following cardiac surgery [27].
Streptokinase
Streptokinase binds plasminogen to form plasmin. Nebulized streptokinase improved oxygenation and lung compliance in a phase 3 trial in 60 patients with late phase (> 10 days) severe ARDS, suggesting promise as a rescue therapy for ARDS patients [28].
Potential therapies in preclinical ARDS studies
There are a substantial number of potential therapies in preclinical testing. We will concentrate on those demonstrating particular promise in each of the key therapeutic target areas (Table 1, Fig. 3).
Pleiotropic immunomodulators
Elafin
Elafin is an endogenous and immunomodulatory protease inhibitor produced by lung epithelial cells among others. Low levels of elafin, due to dysregulated cleavage, are associated with high mortality in ARDS [29,30,31]. One study showed that a functional variant of elafin that was more resistant to degradation had enhanced therapeutic benefit in a mouse model of LPS-induced ALI [30]. Specifically, it dampened immune cell infiltration into the lung and lowered monocyte chemoattractant protein (MCP)-1 levels [30].
Alpha 1-antitrypsin
Alpha 1-antitrypsin (AAT) is an endogenous protease inhibitor of several pro-inflammatory cytokines associated with ARDS including interleukin-6, IL-1β, and TNF-α. AAT inactivation has been demonstrated in infected lung lobes in community-acquired pneumonia [32]. AAT significantly improved oxygenation, decreased pulmonary edema and BAL protein levels and inflammatory cytokines, and inhibited cell apoptosis in a dual-hit mechanical ventilation and LPS-induced ALI rodent model [33]. Another study using the same dual-hit injury model in the rat (and a single-hit murine model) found no therapeutic benefit with AAT treatment [34], suggesting that additional studies are needed to further understand its therapeutic potential.
Pathway-specific immunomodulators
Imatinib
The tyrosine kinase inhibitor imatinib has potent antioxidant and anti-inflammatory effects in vivo and has been shown to ameliorate lung injury and mortality in single- and dual-hit ARDS preclinical models [35, 36]. There is also an ongoing ‘first-in-human study’ examining the effects of imatinib in healthy volunteers exposed to LPS with no results available yet (NCT03328117).
Bevacizumab
Bevacizumab, a human monoclonal antibody against vascular endothelial growth factor (VEGF), has been investigated in a model of high-permeability pulmonary edema in mice, which was induced by VEGF overexpression [37]. Bevacizumab was shown to reduce lung fluid and BAL protein levels [37]. Currently, there is a phase 2/3 trial recruiting patients with SARS-CoV-2 pneumonia for treatment with bevacizumab (NCT04275414).
Anti-IFN-γ
Interferons appear to play a complex role in ARDS, with variable effects reported depending on the specific interferon, whether type I, II or III, and ARDS etiologic agent. Interferon-β1α (Type I interferon), which has anti-viral, anti-inflammatory, and anti-fibrotic functions demonstrated promise in a phase 2a study, but the subsequent phase 3 study did not show efficacy in ARDS [38]. In contrast, certain interferons may worsen influenza-induced ARDS, as evidenced by the finding that a monoclonal antibody to IFN-γ (Type II interferon) reduced the severity of murine H1N1 influenza-induced ARDS, reduced inflammation, and improved mortality [39]. Interestingly, a recent study by Ziegler et al. showed that IFN-γ upregulates ACE2 expression in lung epithelial cells and hence could aid SARS-CoV-2 viral entry [40]. Anti-IFN-γ therapy may have potential as a therapy for COVID-19.
NLRP3 inflammasome inhibitors
The NLRP3 inflammasome is important in innate immunity and causes caspase 1 activation and the release of pro-inflammatory cytokines such as IL-1β [41]. Pirfenidone, a NLRP3 inflammasome inhibitor, was shown to suppress oxidative stress and apoptosis in vitro [42]. In a LPS-induced ALI mouse model, pirfenidone reduced lung injury scores, lung cell infiltration, and lung permeability, while also limiting caspase activation, inflammatory IL-1β release and profibrotic, TGF-β release [42]. In a recently published abstract, tetracycline, another NLRP3 inflammasome inhibitor, was shown to reduce mortality, vascular leakage, and neutrophil infiltration in a murine LPS ALI model [43]. Caspase activation and pro-inflammatory cytokine release were also diminished [43]. Currently, pirfenidone is under phase 3 clinical investigation in the treatment of SARS-CoV-2 (NCT04282902).
Targeting epithelial/endothelial dysfunction
TRPV4 inhibitors
The transient receptor potential vanilloid 4 (TRPV4) channel is a mechano-sensitive and immuno-sensitive calcium transport channel which functions to maintain pulmonary epithelial cell homeostasis. Increased TRPV4 channel activity has been implicated in ARDS pathology particularly in the context of lung stiffness [44, 45], leading to alveolar epithelial and endothelial barrier dysfunction, activation of innate immune cells, and potentiation of pro-inflammatory cytokine release, oxidative stress, and extracellular matrix deposition [45, 46]. TRPV4 −/− mice are protected against VILI [47] and chemically induced ALI [44], while TRPV4 channel inhibitors GSK2220691 and GSK2337429A also reduced ALI [44]. The TRPV4 inhibitors, GSK634775 and GSK1016790, attenuated acid instillation or chlorine gas-induced lung injury, decreasing lung edema, improving oxygenation, and attenuating immune cell infiltration and pro-inflammatory cytokine release [48]. However, a recent first-in-human study of TRPV4 inhibitor, GSK2798745, in volunteers receiving inhaled LPS was terminated early for inefficacy (NCT03511105). The effect of TRPV4 appears cell and injury specific, affecting its utility as a therapeutic target, as recently, macrophage TRPV4 activity has been shown to enhance macrophage phagocytosis and to confer protection against Pseudomonas aeruginosa infection in mice [49].
Adenosine A2A receptor agonists
Adenosine A2A receptors which are expressed on many cell types have been shown to regulate fluid transport as well as inflammation in the lung [50]. The adenosine A2A receptor agonist GW328267C enhanced alveolar fluid clearance in models of acid instillation, LPS, and live E.coli-induced lung injury [50]. Another adenosine A2A receptor agonist, CGS-21680, improved lung compliance and reduced neutrophil infiltration and pro-inflammatory cytokine release in a rat VILI model [51].
RAGE inhibitors
The receptor for advanced glycation end-products (RAGE) is expressed primarily in alveolar type-1 epithelial cells and is a regulator of epithelial barrier transport. Plasma-soluble RAGE concentrations constitute a marker of epithelial lung injury, are increased in ARDS patients, and can predict ARDS development in ‘at risk’ patients [52]. RAGE appears to drive lung injury also, as evidenced by the finding that blockade of RAGE (using peptides, monoclonal antibodies, or soluble RAGE decoy receptors) reduced acid-induced lung injury in mice [53] and piglets [54].
Haptoglobin
Plasma-free hemoglobin causes the formation of reactive oxygen species and is elevated in clinical pneumonia or sepsis. Scavengers of plasma-free hemoglobin such as haptoglobin reduced iron availability, oxidative injury, and lung injury and increased survival in a preclinical model of S. aureus pneumonia [55]. Transgenic mice overexpressing haptoglobin were also protected from hemoglobin-included lung injury [56].
Pro-resolution effects
Lipoxin A4
Lipoxin A4, which is an endogenous pro-resolving lipid mediator, enhanced alveolar epithelial wound repair, promoted differentiation of alveolar type II (ATII) cells to type I cells, promoted ATII proliferation and limited apoptosis in vitro [57]. In a murine LPS-induced ALI model, lipoxin A4 enhanced alveolar epithelial type II cell proliferation, decreased apoptosis by limiting caspase 3 activation and limited epithelial–mesenchymal transition as evidenced by immunofluorescent staining [58]. Lipoxin A4 warrants further investigation in other preclinical ARDS models.
Emerging therapies for COVID-19-induced ARDS
The lack of proven therapies for COVID-19 ARDS has prompted a vast research effort to identify new targets or repurpose existing drugs to treat COVID-19-induced ARDS (Table 2). There are two distinct strategies being pursued, namely strategies that are targeted at the virus itself (reducing replication, ACE-2 receptor binding, etc.) and strategies that modulate the host immune response to the virus infection (targeted or nonspecific immune-modulating drugs). Much of the data available in studies to date come from clinical case series, retrospective analyses, or uncontrolled clinical trials, and so definitive proof of efficacy for interventions is lacking. Nevertheless, given the urgent need for information on which to base treatment decisions, we have included such studies where better designed studies are lacking.
A positive effect of this global focus on severe COVID-19 disease should be the acceleration of multiple potential therapies into clinical testing. Given the rapidly evolving nature of COVID-19 research, we indicate where have cited unpublished and/or un-reviewed reports in this section.
Antiviral therapies/strategies
Remdesivir
Remdesivir, a broad-spectrum antiviral originally investigated as an anti-Ebola drug [59], is an analogue of adenosine that disrupts viral RNA polymerase and viral replication [60]. Remdesivir inhibits MERS-CoV and SARS-CoV in vitro and in vivo [60]. A recent study showed that remdesivir was particularly effective against SARS-CoV-2 infection in vitro [61]. A study of compassionate remdesivir use in 61 patients with SARS-CoV-2 infection observed clinical improvement in 68% of cases with improved oxygenation and a decrease in patients requiring mechanical ventilation [62]. An unpublished recent report suggesting that remdesivir shortened recovery times but did not impact mortality rates has led to the drug being licensed for use in COVID-19 patients in the USA. A recently completed phase 3 study of 237 COVID-19 patients in China showed no significant improvement in clinical outcomes although there was a trend for enhanced recovery time with remdesivir treatment [63]. Most recently, a randomized, blinded, placebo controlled trial in over 1000 patients demonstrated that Remdesivir shortened the time to recovery in adults hospitalized with COVID-19 and evidence of lower respiratory tract infection [64]. The results of several other phase 2/3 remdesivir clinical trials are awaited (Table 2).
Favipiravir
Favipiravir is a broad-spectrum antiviral RNA polymerase inhibitor, already approved for use in influenza A and B [65]. A recent, open-label, control study, showed that favipiravir exhibited significant improvements in chest CT scans and viral clearance in COVID-19 patients [66]. Several other clinical studies are underway with one examining the potential of favipiravir in combination with tocilizumab.
Lopinavir/ritonavir
Lopinavir/ritonavir are HIV protease inhibitors and are generally used as part of combination therapies. A recently concluded, open-label trial of lopinavir/ritonavir in 199 severe COVID-19 patients unfortunately showed no clinical improvement, although the mortality rate was slightly lower in the treatment group (19.2% vs. 25%) [67]. Potential explanations include lopinavir/ritonavir use in late COVID-19 infection, its use as a single agent, and in relatively lower doses, which should be addressed in ongoing studies [67]. Of relevance, another recently completed phase 2 study showed that early combined treatment of lopinavir/ritonavir with IFN-β1β and ribavirin reduced viral shedding and shortened hospital stays compared to lopinavir/ritonavir alone in mild–moderate COVID-19 patients [68].
Umifenovir
Umifenovir (also known as arbidol), an antiviral approved for influenza that can affect viral interaction and binding via ACE2, was recently shown to enhance viral clearance in comparison with lopinavir/ritonavir treatment, in a retrospective study of 50 COVID-19 patients [69]. An un-reviewed preprint reporting an open-label, multicenter trial comparing arbidol with favipiravir in 240 COVID-19 patients, with recovery at day 7 as the primary outcome measure, found no differences between these two treatments [70]. A number of studies are currently examining the safety and efficacy of arbidol in patients with COVID-19.
Chloroquine and hydroxychloroquine
The antimalarial drugs, chloroquine and its hydroxylated version, hydroxychloroquine, disrupt ACE2 binding and hence viral entry and also affect endosomal and lysosomal pH, which can inhibit the virus from merging with host cells [71]. These drugs also suppress pro-inflammatory cytokine release [72]. Chloroquine has specifically been shown to inhibit influenza A H5N1 virus-induced lung injury in preclinical models [73] and SARS-CoV-2 infection in vitro [61]. A small clinical study recently showed that hydroxychloroquine in combination with azithromycin reduced viral load in 20 patients with SARS-CoV-2 infection [74]. Conversely, concerns have been raised regarding potential adverse effects (e.g., cardiotoxicity) with chloroquine and hydroxychloroquine, particularly at high doses and when used in combination with azithromycin, in COVID-19 patients [75, 76]. A major observational study in 14,888 COVID-19 patients treated with either hydroxychloroquine or chloroquine, alone or in combination with a macrolide, found that these patients had an increased risk of mortality and an increased risk of de novo ventricular arrhythmia [77]. A number of other and larger clinical investigations of chloroquine and hydroxychloroquine, alone or in combination with other antivirals, are underway (Table 2).
TMPRSS2 inhibitor
SARS-CoV-2 viral entry into lung epithelial cells is dependent on the ACE2 receptor, while priming of the viral spike protein is dependent on the host serine protease TMPRSS2 [78]. A protease inhibitor of TMPRSS2 blocked viral entry in vitro and may be a promising therapeutic option [78]. Clinical studies investigating the efficacy of TMPRSS2 inhibitor, camostat mesilate, are currently recruiting.
Baricitinib
Another drug which may inhibit viral entry via ACE2 receptor-mediated endocytosis is baricitinib, a JAK inhibitor, that also disrupts the cytokine cascade and dampens inflammation [79] and is an approved drug for rheumatoid arthritis. Baricitinib with its anti-inflammatory and antiviral potential was identified using a data search with the BenevolentAI drug discovery platform. A recent study of 12 patients with moderate COVID-19 observed that baricitinib administered at 4 mg/day for 14 days was well tolerated and improved outcome in these patients when compared to patients receiving standard care [80]. Other larger trials evaluating baricitinib for COVID-19 are underway.
Convalescent plasma
Hoffmann et al. showed that SARS-CoV-1 serum from convalescent patients offered protection from SARS-CoV-2 infection, and this option may perhaps be effective if used prophylactically [78]. Convalescent plasma has also been shown to reduce viral load and mortality in critically ill H1N1 patients [81] and most recently has been shown to reduce viral load and improve outcome in a series of 5 cases of critically ill SARS-CoV-2 patients [82]. An un-reviewed preprint of the results from a large expanded access trial of 5000 COVID-19 patients treated with convalescent plasma (NCT04338360) showed that treatment was well tolerated [83]. Other trials assessing the safety and efficacy of anti-SARS-CoV-2-inactivated convalescent plasma in COVID-19 patients are underway.
Angiotensin II
SARS-CoV-2 binds to the ACE receptor on lung epithelial cells, which is a key step in virus infection of these cells. This also leads to a decrease in ACE2 and an increase in detrimental angiotensin II. Losartan, which is an angiotensin II receptor antagonist, is currently under investigation in SARS-CoV-2 patients (NCT04328012).
Immunomodulatory—pleiotropic effects
Methylprednisolone
The role of steroids indications for COVID-19 patients is unclear, with effects reported that might be harmful or beneficial depending on the specific clinical context [84,85,86]. Some evidence suggests that steroid use may hinder viral clearance in MERS coronavirus infection [87]. However, the effects of steroids in COVID-19 appear to depend on the dose and the degree of ‘hyperinflammation’ present, the stage of infection, and the presence of ARDS [85, 86]. A recent single-center, retrospective study of 46 patients with COVID-19 published as an un-reviewed preprint showed that early, low-dose, and short-term administration of methylprednisolone improved chest CT and clinical outcome in the treatment group [88]. Another larger retrospective study of 201 COVID-19 patients showed that methylprednisolone treatment in those with ARDS reduced the risk of death [89]. Currently, there are a number of phase 2/3 clinical trials investigating the efficacy and safety of methylprednisolone in patients with COVID-19 ARDS. Hopefully, these studies should provide clarity on the role of steroids in these patients. The recent press release suggesting a mortality benefit for Dexamethasone in COVID-19 patients in the RECOVERY trial is of particular interest in this regard.
Thalidomide
Thalidomide, an immunomodulatory drug that acts to enhance apoptosis, inhibits IL-6 and promotes T cell responses and has been shown to lead beneficial effects in preclinical bacterial- and viral-induced ARDS [90, 91]. Clinical phase 2 investigations of thalidomide for therapy against SARS-CoV-2 infection are underway.
Interferons
As discussed earlier, type I interferon, interferon-β1α, was ineffective as a sole agent in a recent phase 3 ARDS trial ARDS [38]. However, type I interferons have been shown to respond with different inhibitory potencies toward MERS and SARS [92] and, as such, interferons have been investigated, as an adjunct to antivirals, in these viral infections [93]. A recent study published as an un-reviewed preprint has observed that SARS-CoV-2 infection is potentially sensitive to type I interferons [94]. As mentioned previously, a recent phase 2 study of triple therapy with lopinavir/ritonavir, ribavirin, and IFN-β1β enhanced the recovery of patients with SARS-CoV-2 infection compared to lopinavir/ritonavir alone [68]. There are a number of other phase 2/3 clinical trials investigating the efficacy of both type I or type III interferons (including REMAP-CAP, DisCoVeRy, and SOLIDARITY), either as sole agents or as co-therapies in patients with SARS-CoV-2 (Table 2).
Mesenchymal stromal cell (MSC) therapies
The immunomodulatory effects of MSCs have generated considerable interest as a potential therapeutic for COVID-19 ARDS. A recent study of 7 COVID-19 patients observed that a single dose of ACE2-/- MSCs (10 million cells/kg) was well tolerated and improved pulmonary function, reduced TNF-α release while enhancing IL-10 release in comparison with the placebo [95]. A number of other trials are investigating the effects of MSCs and MSC-derived exosomes in patients with SARS-CoV-2 infection (Table 2).
Immunomodulatory—pathway specific
A subgroup of severely ill COVID-19 patients develop a ‘cytokine storm’ profile with rapid and sustained elevations in cytokines such as IL-6, and fulminant organ failure with features in common with secondary hemophagocytic lymphohistiocytosis (HLH) [96]. This has led to interest in specific anti-cytokine therapies.
Tocilizumab and sarilumab
Tocilizumab and sarilumab are human monoclonal antibodies that block the IL-6 receptor. IL-6 inhibition has been shown to be therapeutic in patients with adult-onset Still’s disease complicated with SIRS and ARDS [97, 98]. One recent non-controlled retrospective study of 21 patients with COVID-19, published as an un-reviewed preprint, suggested that tocilizumab treatment may have decreased white cell counts and improved CT lung opacity and lung oxygenation [99]. There are currently several phase 2/3 trials investigating tocilizumab and/or sarilumab for COVID-19 patients, with reports expected imminently.
Anakinra
Anakinra is a recombinant IL-1 receptor antagonist that neutralizes the biologic activity of IL-1a and IL-1b by competitively inhibiting their binding to interleukin-1 type I receptor and is widely used in rheumatic diseases. Anakinra did not improve mortality in patients with sepsis and septic shock in large phase 3 studies [100,101,102]. However, in a post hoc analysis anakinra improved survival in the subgroup of sepsis patients with features of HLH (ferritin elevation in excess of 2000 ng/ml, coagulopathy, and liver enzyme elevations) [103]. Anakinra is being trialled in the ‘COVID domain’ of the REMAP-CAP study (NCT02735707).
Other potential therapies
Heparin
Disordered coagulation, specifically, pulmonary microvascular thrombosis is increasingly implicated in the pathogenesis of severe COVID-19 respiratory failure. Other thrombotic complications including deep venous thrombosis are also reported. Anticoagulant therapy, mainly with low molecular weight heparin, has been associated with better prognosis in severe COVID-19 patients with evidence of coagulation activation such as markedly elevated D-dimers [104]. Consequently, heparin has been recommended by some expert consensus groups; however, its efficacy remains to be proven. Intravenous heparin is being trialled in the REMAP-CAP study (NCT02735707). Studies of nebulized heparin, such as the CHARTER study, are also in progress [105].
Finding ARDS therapies—future directions
Improved preclinical models
Understanding and, where relevant, addressing limitations to current preclinical models may help reduce future ‘translational failures’ of potential therapies for ARDS. Preclinical models are designed to be reliable and reproducible but, in achieving this, may poorly model the complexity of ARDS. More clinically relevant experimenal models can provide initial proof-of-principle; it allows ineffective strategies to be rapidly discarded.
Issues such as multiple or sequential insults, the timing of insults, the role of host factors such as age, sex, and premorbid conditions, and the usually prolonged duration of ARDS are not well reflected in current preclinical models. Testing promising therapies in more complex and diverse animal models, of varying age and species, employing multiple hits, and modeling longer durations of ARDS, while challenging, may be a useful step prior to embarking on clinical studies. Multicenter trials, incorporating randomization and blinding for preclinical studies, may minimize bias and improve robustness by increasing heterogeneity.
Other useful ‘intermediate’ steps for promising therapies prior to trials in ARDS patients may be the use of human models such as endotoxin inhalation in volunteers or testing in surgical populations, such as those undergoing one lung ventilation. Testing promising therapies in the ex vivo human lung perfusion model may provide proof of concept that the intervention can work in an acutely injured human lung.
Improved clinical trials
Improving our approach to clinical trial design and patient selection [106] may enhance the likelihood of finding effective therapies. One key issue relates to the heterogeneity of ARDS and the nonspecific nature of the ARDS clinical criteria, which may result in recruitment of patients who do not possess the underlying injury processes and biologic pathways characteristic of ARDS. ‘Practical enrichment’ involves careful selection of candidates who are likely to complete the intervention and survive the study period. ‘Prognostic enrichment’ aims to reduce the numbers required to detect a significant difference by enrolling patients who are most likely to experience the primary endpoint. ‘Predictive enrichment’ involves selecting patients based on pathobiological factors that will predispose them to a treatment response. This latter approach may offer most promise, by selecting for patients who have a strong likelihood for a response to the intervention (and by the same token, select ‘out’ those who are unlikely to respond). This would reduce study noise, sample size, and study-associated harm. In ARDS, this approach has already borne fruit: an important—positive—study of prone positioning randomized only patients who demonstrated an initial positive response to prone positioning. The use of adaptive clinical trial designs, which permits modifications of the trial and/or statistical procedures after its initiation, e.g., to favor recruitment to intervention arms where favorable outcome data appears to be emerging, may also enhance the potential to identify effective interventions. The REMAP-CAP trial is an example of such a trial in a relevant clinical population.
Targeting ARDS subtypes
Identifying patients more likely to respond to a specific pharmacologic intervention should increase chances of trial success. A key recent advance in our understanding of the pathobiology of ARDS has been the ability to divide ARDS into subgroups or sub-phenotypes. Latent class analysis identifies one-third of ARDS patients with a ‘hyper-inflammatory’ phenotype, and reanalysis of a large negative RCT of simvastatin in ARDS using this approach suggested benefit in the ‘hyperinflammatory’ group [107]. ARDS phenotyping based on the focal versus diffuse distribution of lung infiltrates is also potentially feasible [108], as are transcriptomics-based approaches [109]. While prospective trials are required to validate phenotyping approaches, and subsequently to test therapies in specific phenotypes, this approach offers considerable hope for the repurposing of drugs previously deemed to have ‘failed’ clinical translation.
Conclusions
There is a host of potential drug therapies demonstrating promise for ARDS, from drugs that modulate the immune response, specific inflammatory pathway blockers, epithelial and channel function modulators, endothelial and vascular dysfunction therapies, anticoagulant drugs, and therapies that aid resolution of ARDS. A promising pipeline of therapies is also progressing through preclinical testing. An important area of investigation is the potential for advances in our understanding of the pathobiology of ARDS and specifically the potential to identify biologically homogenous subtypes within ARDS, to enable us to target more specific therapies. It is hoped that the substantial number of studies globally investigating potential therapies for severe COVID-19 patients will help the identification of effective therapies for ARDS.
References
Ashbaugh DG, Bigelow DB, Petty TL, Levine BE (1967) Acute respiratory distress in adults. Lancet 2:319–323
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800
Laffey JG, Kavanagh BP (2018) Negative trials in critical care: why most research is probably wrong. Lancet Respir Med 6:659–660
Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, Martin-Rodriguez C, Diaz-Dominguez FJ, Serna-Grande P, Rivas R, Ferreres J, Belda J, Capilla L, Tallet A, Anon JM, Fernandez RL, Gonzalez-Martin JM (2020) Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 8:267–276
Ji M, Chen T, Wang B, Chen M, Ding Q, Chen L, Fang Y, Yu X, Chen Y, Wang X, He Y, Jiang Y (2018) Effects of ulinastatin combined with mechanical ventilation on oxygen metabolism, inflammation and stress response and antioxidant capacity of ARDS. Exp Ther Med 15:4665–4670
Fowler AA 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, Thacker LR 2nd, Natarajan R, Brophy DF, Sculthorpe R, Nanchal R, Syed A, Sturgill J, Martin GS, Sevransky J, Kashiouris M, Hamman S, Egan KF, Hastings A, Spencer W, Tench S, Mehkri O, Bindas J, Duggal A, Graf J, Zellner S, Yanny L, McPolin C, Hollrith T, Kramer D, Ojielo C, Damm T, Cassity E, Wieliczko A, Halquist M (2019) Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA 322:1261–1270
Ryter SW, Otterbein LE, Morse D, Choi AM (2002) Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem 234–235:249–263
Jahn N, Lamberts RR, Busch CJ, Voelker MT, Busch T, Koel-Simmelink MJ, Teunissen CE, Oswald DD, Loer SA, Kaisers UX, Weimann J (2015) Inhaled carbon monoxide protects time-dependently from loss of hypoxic pulmonary vasoconstriction in endotoxemic mice. Respir Res 16:119
Fredenburgh LE, Perrella MA, Barragan-Bradford D, Hess DR, Peters E, Welty-Wolf KE, Kraft BD, Harris RS, Maurer R, Nakahira K, Oromendia C, Davies JD, Higuera A, Schiffer KT, Englert JA, Dieffenbach PB, Berlin DA, Lagambina S, Bouthot M, Sullivan AI, Nuccio PF, Kone MT, Malik MJ, Porras MAP, Finkelsztein E, Winkler T, Hurwitz S, Serhan CN, Piantadosi CA, Baron RM, Thompson BT, Choi AM (2018) A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight 3(23):e124039
Devaney J, Horie S, Masterson C, Elliman S, Barry F, O'Brien T, Curley GF, O'Toole D, Laffey JG (2015) Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 70:625–635
Horie S, Gaynard S, Murphy M, Barry F, Scully M, O'Toole D, Laffey JG (2020) Cytokine pre-activation of cryopreserved xenogeneic-free human mesenchymal stromal cells enhances resolution and repair following ventilator-induced lung injury potentially via a KGF-dependent mechanism. Intensive Care Med Exp 8:8
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA (2014) Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med 3:24–32
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD (2018) Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med 7:154–162
Yip HK, Fang WF, Li YC, Lee FY, Lee CH, Pei SN, Ma MC, Chen KH, Sung PH, Lee MS (2020) Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med 48:e391–e399
Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, Young D, Bentley A, McVerry B, Wunderink RG, Doerschug KC, Summers C, Rojas M, Jenkins ED, Ting A (2019) Primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative advanced therapy medicinal product (ATMP), in acute respiratory distress syndrome (MUST-ARDS)B14 late breaking clinical trials, pp A7353–A7353
Christie JD, Vaslef S, Chang PK, May AK, Gunn SR, Yang S, Hardes K, Kahl L, Powley WM, Lipson DA, Bayliffe AI, Lazaar AL (2015) A Randomized dose-escalation study of the safety and anti-inflammatory activity of the p38 mitogen-activated protein kinase inhibitor dilmapimod in severe trauma subjects at risk for acute respiratory distress syndrome. Crit Care Med 43:1859–1869
Proudfoot A, Bayliffe A, O'Kane CM, Wright T, Serone A, Bareille PJ, Brown V, Hamid UI, Chen Y, Wilson R, Cordy J, Morley P, de Wildt R, Elborn S, Hind M, Chilvers ER, Griffiths M, Summers C, McAuley DF (2018) Novel anti-tumour necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax 73:723–730
Krenn K, Lucas R, Croize A, Boehme S, Klein KU, Hermann R, Markstaller K, Ullrich R (2017) Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit Care 21:194
Ware LB, Magarik JA, Wickersham N, Cunningham G, Rice TW, Christman BW, Wheeler AP, Bernard GR, Summar ML (2013) Low plasma citrulline levels are associated with acute respiratory distress syndrome in patients with severe sepsis. Crit Care 17:R10
Klein N, Gembardt F, Supe S, Kaestle SM, Nickles H, Erfinanda L, Lei X, Yin J, Wang L, Mertens M, Szaszi K, Walther T, Kuebler WM (2013) Angiotensin-(1–7) protects from experimental acute lung injury. Crit Care Med 41:e334–e343
Zambelli V, Bellani G, Borsa R, Pozzi F, Grassi A, Scanziani M, Castiglioni V, Masson S, Decio A, Laffey JG, Latini R, Pesenti A (2015) Angiotensin-(1–7) improves oxygenation, while reducing cellular infiltrate and fibrosis in experimental acute respiratory distress syndrome. Intensive Care Med Exp 3:44
Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, Poirier G, Ronco JJ, Tidswell M, Hardes K, Powley WM, Wright TJ, Siederer SK, Fairman DA, Lipson DA, Bayliffe AI, Lazaar AL (2017) A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 21:234
Bastarache JA, Fremont RD, Kropski JA, Bossert FR, Ware LB (2009) Procoagulant alveolar microparticles in the lungs of patients with acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 297:L1035–1041
Morris PE, Steingrub JS, Huang BY, Tang S, Liu PM, Rhode PR, Wong HC (2012) A phase I study evaluating the pharmacokinetics, safety and tolerability of an antibody-based tissue factor antagonist in subjects with acute lung injury or acute respiratory distress syndrome. BMC Pulm Med 12:5
Camprubi-Rimblas M, Tantinya N, Guillamat-Prats R, Bringue J, Puig F, Gomez MN, Blanch L, Artigas A (2019) Effects of nebulized antithrombin and heparin on inflammatory and coagulation alterations in an acute lung injury model in rats. J Thromb Haemost 18:571–583
Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ (2010) Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care 14:R180
Dixon B, Smith R, Santamaria JD, Orford NR, Wakefield BJ, Ives K, McKenzie R, Zhang B, Yap CH (2015) A trial of nebulised heparin to limit lung injury following cardiac surgery. Anaesth Intensive Care 44:28–33
Abdelaal Ahmed Mahmoud A, Mahmoud HE, Mahran MA, Khaled M (2019) Streptokinase versus unfractionated heparin nebulization in patients with severe acute respiratory distress syndrome (ARDS): a randomized controlled trial with observational controls. J Cardiothorac Vasc Anesth 34:436–443
Kerrin A, Weldon S, Chung AH, Craig T, Simpson AJ, O'Kane CM, McAuley DF, Taggart CC (2013) Proteolytic cleavage of elafin by 20S proteasome may contribute to inflammation in acute lung injury. Thorax 68:315–321
Small DM, Zani ML, Quinn DJ, Dallet-Choisy S, Glasgow AM, O'Kane C, McAuley DF, McNally P, Weldon S, Moreau T, Taggart CC (2015) A functional variant of elafin with improved anti-inflammatory activity for pulmonary inflammation. Mol Ther 23:24–31
Wang T, Zhu Z, Liu Z, Yi L, Yang Z, Bian W, Chen W, Wang S, Li G, Li A, Martin GS, Zhu X (2017) Plasma neutrophil elastase and elafin as prognostic biomarker for acute respiratory distress syndrome: a multicenter survival and longitudinal prospective observation study. Shock 48:168–174
Greene C, Taggart C, Lowe G, Gallagher P, McElvaney N, O’Neill S (2003) Local impairment of anti-neutrophil elastase capacity in community-acquired pneumonia. J Infect Dis 188:769–776
Wang X, Gong J, Zhu J, Jin Z, Gao W (2019) Alpha 1-antitrypsin for treating ventilator-associated lung injury in acute respiratory distress syndrome rats. Exp Lung Res 45:209–219
Juschten J, Ingelse SA, Maas MAW, Girbes ARJ, Juffermans NP, Schultz MJ, Tuinman PR (2019) Antithrombin plus alpha-1 protease inhibitor does not affect coagulation and inflammation in two murine models of acute lung injury. Intensive Care Med Exp 7:36
Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JG, Letsiou E, Dudek SM (2015) Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 309:L1294–1304
Stephens RS, Johnston L, Servinsky L, Kim BS, Damarla M, (2015) The tyrosine kinase inhibitor imatinib prevents lung injury and death after intravenous LPS in mice. Physiol Rep 3(11)
Watanabe M, Boyer JL, Crystal RG (2009) Genetic delivery of bevacizumab to suppress vascular endothelial growth factor-induced high-permeability pulmonary edema. Hum Gene Ther 20:598–610
Ranieri VM, Pettila V, Karvonen MK, Jalkanen J, Nightingale P, Brealey D, Mancebo J, Ferrer R, Mercat A, Patroniti N, Quintel M, Vincent JL, Okkonen M, Meziani F, Bellani G, MacCallum N, Creteur J, Kluge S, Artigas-Raventos A, Maksimow M, Piippo I, Elima K, Jalkanen S, Jalkanen M, Bellingan G; Group IS (2020) Effect of intravenous interferon beta-1a on death and days free from mechanical ventilation among patients with moderate to severe acute respiratory distress syndrome: a randomized clinical trial. JAMA https:doi.org/10.1001/jama.2019.22525
Liu B, Bao L, Wang L, Li F, Wen M, Li H, Deng W, Zhang X, Cao B (2019) Anti-IFN-gamma therapy alleviates acute lung injury induced by severe influenza A (H1N1) pdm09 infection in mice. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2019.07.009
Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J (2020) SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. https://doi.org/10.1016/j.cell.2020.04.035
Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA (2015) Critical role for the NLRP3 inflammasome during acute lung injury. J Immunol 192:5974–5983
Li Y, Li H, Liu S, Pan P, Su X, Tan H, Wu D, Zhang L, Song C, Dai M, Li Q, Mao Z, Long Y, Hu Y, Hu C (2018) Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol 99:134–144
Bode C, Peukert K, Schewe J-C, Putensen C, Latz E, Steinhagen F, (2019) Tetracycline alleviates acute lung injury by inhibition of NLRP3 inflammasome. Eur Respir J 54:PA2175
Morty RE, Kuebler WM (2014) TRPV4: an exciting new target to promote alveolocapillary barrier function. Am J Physiol Lung Cell Mol Physiol 307:L817–821
Michalick L, Kuebler WM (2020) TRPV4—a missing link between mechanosensation and immunity. Front Immunol. https://doi.org/10.3389/fimmu.2020.00413
Scheraga RG, Southern BD, Grove LM, Olman MA (2017) The role of transient receptor potential vanilloid 4 in pulmonary inflammatory diseases. Front Immunol 8:503
Hamanaka K, Jian M-Y, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC (2010) TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299:L353–L362
Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE (2014) TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol 307:L158–172
Scheraga RG, Abraham S, Grove LM, Southern BD, Crish JF, Perelas A, McDonald C, Asosingh K, Hasday JD, Olman MA (2020) TRPV4 protects the lung from bacterial Pneumonia via MAPK molecular pathway switching. J Immunol 204:1310–1321
Hans GF, Stephanie RK, David AL, Michael AM, Mark AS (2012) The adenosine 2A receptor agonist GW328267C improves lung function after acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 303:L259–L271
Chen CM, Penuelas O, Quinn K, Cheng KC, Li CF, Zhang H, Slutsky AS (2009) Protective effects of adenosine A2A receptor agonist in ventilator-induced lung injury in rats. Crit Care Med 37:2235–2241
Jabaudon M, Berthelin P, Pranal T, Roszyk L, Godet T, Faure JS, Chabanne R, Eisenmann N, Lautrette A, Belville C, Blondonnet R, Cayot S, Gillart T, Pascal J, Skrzypczak Y, Souweine B, Blanchon L, Sapin V, Pereira B, Constantin JM (2018) Receptor for advanced glycation end-products and ARDS prediction: a multicentre observational study. Sci Rep 8:2603
Blondonnet R, Audard J, Belville C, Clairefond G, Lutz J, Bouvier D, Roszyk L, Gross C, Lavergne M, Fournet M, Blanchon L, Vachias C, Damon-Soubeyrand C, Sapin V, Constantin JM, Jabaudon M (2017) RAGE inhibition reduces acute lung injury in mice. Sci Rep 7:7208
Audard J, Godet T, Blondonnet R, Joffredo JB, Paquette B, Belville C, Lavergne M, Gross C, Pasteur J, Bouvier D, Blanchon L, Sapin V, Pereira B, Constantin JM, Jabaudon M (2019) Inhibition of the receptor for advanced glycation end-products in acute respiratory distress syndrome: a randomised laboratory trial in piglets. Sci Rep 9:9227
Remy KE, Cortes-Puch I, Solomon SB, Sun J, Pockros BM, Feng J, Lertora JJ, Hantgan RR, Liu X, Perlegas A, Warren HS, Gladwin MT, Kim-Shapiro DB, Klein HG, Natanson C (2018) Haptoglobin improves shock, lung injury, and survival in canine pneumonia. JCI Insight. https://doi.org/10.1172/jci.insight.123013
Yang F, Haile DJ, Berger FG, Herbert DC, Van Beveren E, Ghio AJ (2003) Haptoglobin reduces lung injury associated with exposure to blood. Am J Physiol Lung Cell Mol Physiol 284:L402–409
Zheng S, D'Souza VK, Bartis D, Dancer RC, Parekh D, Naidu B, Gao-Smith F, Wang Q, Jin S, Lian Q, Thickett DR (2016) Lipoxin A4 promotes lung epithelial repair whilst inhibiting fibroblast proliferation. ERJ Open Res 2(3)
Yang JX, Li M, Chen XO, Lian QQ, Wang Q, Gao F, Jin SW, Zheng SX (2019) Lipoxin A4 ameliorates lipopolysaccharide-induced lung injury through stimulating epithelial proliferation, reducing epithelial cell apoptosis and inhibits epithelial-mesenchymal transition. Respir Res 20:192
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S (2016) Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 531:381–385
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS (2017) Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aal3653
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 30:269–271
Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure FX, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D'Arminio Monforte A, Ismail S, Kato H, Lapadula G, L'Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T (2020) Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. https://doi.org/10.1056/NEJMoa2007016
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q (2020) Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. https://doi.org/10.1016/S0140-6736(20)31022-9
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fatkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, Members A-SG (2020) Remdesivir for the Treatment of Covid-19 - Preliminary Report. N Engl J Med. https://doi.org/10.1056/NEJMoa2007764
Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci 93:449–463
Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y (2020) Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering. https://doi.org/10.1016/j.eng.2020.03.007
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C (2020) A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382:1787–1799
Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Ng Y-Y, Lo J, Chan J, Tam AR (2020) Triple combination of interferon beta-1b, lopinavir/ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. https://doi.org/10.1016/S0140-6736(20)31042-4
Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, Jianchun XY (2020) Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. https://doi.org/10.1016/j.jinf.2020.03.060
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Ju L, Zhang J, Wang X, (2020) Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv: 2020.2003.2017.20037432
Zhou D, Dai SM, Tong Q (2020) COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. https://doi.org/10.1093/jac/dkaa114
van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL (1997) Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol 24:55–60
Yan Y, Zou Z, Sun Y, Li X, Xu KF, Wei Y, Jin N, Jiang C (2013) Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res 23:300–302
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 105949
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS (2020) Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1834
Borba MGS, Val FFA, Sampaio VS, Alexandre MAAJ, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baãa-da-Silva D, Guerra MVF (2020) Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open 3: e208857–e208857
Mehra MR, Desai SS, Ruschitzka F, Patel AN (2020) Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. https://doi.org/10.1016/S0140-6736(20)31180-6
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S, (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280e8
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395:e30–e31
Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D (2020) Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. https://doi.org/10.1016/j.jinf.2020.04.017
Hung IFN, To KKW, Lee CK, Lee KL, Yan WW, Chan K, Chan WM, Ngai CW, Law KI, Chow FL, Liu R, Lai KY, Lau CCY, Liu SH, Chan KH, Lin CK, Yuen KY (2013) Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest 144:464–473
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L (2020) Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. https://doi.org/10.1001/jama.2020.4783
Joyner M, Wright RS, Fairweather D, Senefeld J, Bruno K, Klassen S, Carter R, Klompas A, Wiggins C, Shepherd JRA, Rea R, Whelan E, Clayburn A, Spiegel M, Johnson P, Lesser E, Baker S, Larson K, Ripoll Sanz J, Andersen K, Hodge D, Kunze K, Buras M, Vogt M, Herasevich V, Dennis J, Regimbal R, Bauer P, Blair J, van Buskirk C, Winters J, Stubbs J, Paneth N, Casadevall A (2020) Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 140200. https://doi.org/10.1172/JCI140200
Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395:683–684
Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, Lagolio E, Celotto S, Pizzol D, Zou L, Tully MA, Ilie PC, Trott M, Lãpez-Sãnchez GF, Smith L (2020) Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med. https://doi.org/10.3389/fmed.2020.00170
Js V, Confalonieri M, Pastores SM, Meduri GU (2020) Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor 2:e0111
Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA, Saudi Critical Care Trial G (2018) Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med 197:757–767
Wang Y, Jiang W, He Q et al (2020) A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Sig Transduct Target Ther 5:57. https://doi.org/10.1038/s41392-020-0158-2
Wu C, Chen X, Cai Y, Xia Ja, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y, (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. https://doi.org/10.1001/jamainternmed.2020.0994
Kumar V, Harjai K, Chhibber S (2010) Thalidomide treatment modulates macrophage pro-inflammatory function and cytokine levels in Klebsiella pneumoniae B5055 induced pneumonia in BALB/c mice. Int Immunopharmacol 10:777–783
Zhu H, Shi X, Ju D, Huang H, Wei W, Dong X (2014) Anti-inflammatory effect of thalidomide on H1N1 influenza virus-induced pulmonary injury in mice. Inflammation 37:2091–2098
Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N (2020) Type 1 interferons as a potential treatment against COVID-19. Antiviral Res 178:104791
Momattin H, Mohammed K, Zumla A, Memish ZA, Al-Tawfiq JA (2013) Therapeutic options for Middle East respiratory syndrome coronavirus (MERS-CoV)–possible lessons from a systematic review of SARS-CoV therapy. Int J Infect Dis 17:e792–798
Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD (2020) SARS-CoV-2 is sensitive to type I interferon pretreatment. J Biol Chem. https://doi.org/10.1074/jbc.AC120.013788
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC (2020) Transplantation of ACE2(−) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis 11:216–228
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Masui-Ito A, Okamoto R, Ikejiri K, Fujimoto M, Tanimura M, Nakamori S, Murata T, Ishikawa E, Yamada N, Imai H, Ito M (2017) Tocilizumab for uncontrollable systemic inflammatory response syndrome complicating adult-onset still disease: case report and review of literature. Medicine (Baltimore) 96:e7596
Morrondo CD, Zarza LP, Gil JG, Pinto Tasende JA, Diez PD, Lopez JM (2016) Benefit of tocilizumab therapy for adult-onset still disease complicated with acute respiratory distress syndrome. J Clin Rheumatol 22:291–293
Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X (2020) Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA 117(20):10970–10975. https://doi.org/10.1073/pnas.2005615117
Fisher CJ Jr, Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL et al (1994) Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA 271:1836–1843
Knaus WA, Harrell FE Jr, LaBrecque JF, Wagner DP, Pribble JP, Draper EA, Fisher CJ Jr, Soll L (1996) Use of predicted risk of mortality to evaluate the efficacy of anticytokine therapy in sepsis. The rhIL-1ra Phase III Sepsis Syndrome Study Group. Crit Care Med 24:46–56
Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 25:1115–1124
Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM (2016) Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 44:275–281
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18:1094–1099
Dixon B, Smith R, Artigas A, Laffey J, McNicholas B, Schmidt E, Nunes Q, Skidmore MA, Andrade de Lome M, Moran J, Van Haren F, Doig G, Gupta S, Ghosh A, Said S, Santamaria J (2020) Can nebulised heparin reduce time to extubation in SARS CoV 2 the CHARTER study protocol. medRxiv:2020.2004.2028.20082552
Shankar-Hari M, Rubenfeld GD (2019) Population enrichment for critical care trials: phenotypes and differential outcomes. Curr Opin Crit Care 25:489–497
Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, McDowell C, Laffey JG, O'Kane CM, McAuley DF (2018) Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 6:691–698
Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, Ferrandiere M, Grelon F, Seguin P, Ichai C, Veber B, Souweine B, Uberti T, Lasocki S, Legay F, Leone M, Eisenmann N, Dahyot-Fizelier C, Dupont H, Asehnoune K, Sossou A, Chanques G, Muller L, Bazin JE, Monsel A, Borao L, Garcier JM, Rouby JJ, Pereira B, Futier E (2019) Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med 7:870–880
Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, Artigas A, Martin-Loeches I, Hoogendijk AJ, van der Poll T, Horn J, Juffermans N, Calfee CS, Schultz MJ (2017) Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 72:876–883
Author information
Authors and Affiliations
Contributions
SH drafted the manuscript; BM and JL wrote the first and subsequent drafts of the manuscript. All authors critically revised the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Horie, S., McNicholas, B., Rezoagli, E. et al. Emerging pharmacological therapies for ARDS: COVID-19 and beyond. Intensive Care Med 46, 2265–2283 (2020). https://doi.org/10.1007/s00134-020-06141-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-020-06141-z