Abstract
In patients with monoclonal gammopathy of undetermined significance (MGUS) the increase of bone turnover rate can increase the risk of fracture. Thus, a treatment normalizing this negative balance could be of benefit in these patients. We studied 100 patients affected by MGUS, grouped according to the presence (group A, 50 patients) or absence (group B) of vertebral fractures and/or osteoporosis. Group A was treated with alendronate (70 mg/weekly) plus calcium and cholecalciferol for 18 months, and group B was treated with calcium and cholecalciferol. After 18 months, the mean bone mineral density (BMD) of the lumbar spine and total femur had increased by 6.1% and 1.5%, respectively, in group A. In the nine patients of this group not taking alendronate, BMD values of the lumbar spine and total femur decreased by 1.6% (P ≤ 0.001 ) and 1.3% (P ≤ 0.01), respectively. In patients of group B, BMD increased by 1.2% at the lumbar spine and decreased by 1.2% at the total femur. Corresponding figures of those patients in the same group not taking calcium and vitamin D supplementation were −0.1% and −1.2%, respectively. At 18 months we observed significant decreases of serum bone markers: the difference between the groups was −23.2 (P ≤ 0.0l) for bone alkaline phosphatase, −23.6 for osteocalcin (P ≤ 0.0l), −35.1 for C-terminal telopeptides of collagen type I (P ≤ 0.00l), and −0.47 for bone sialoprotein (P = nonsignificant). Treatment with alendronate could lead to a significant reduction in fracture risk in MGUS patients with skeletal fragility.
Similar content being viewed by others
Monoclonal gammopathy of undetermined significance (MGUS) is a hematological disorder whose prevalence increases with ageing. In a recent population-based study involving residents of Olmsted County, Minnesota, Kyle and coworkers [1] reported that the prevalence of this condition was 3.2% in persons older than 50 years and 5.3% in those older than 70 years.
Recently, a number of studies have been undertaken in order to evaluate the degree of skeletal involvement in patients affected by MGUS, a disease which often precedes multiple myeloma. Epidemiological studies, in fact, indicate that such patients are at increased risk of fractures, mainly at the axial level [2–4]. Regarding the rate of skeletal turnover, evaluated by the measurement of bone remodeling markers, in most studies it has been possible to demonstrate a mean increase in skeletal resorption markers, which may or may not be associated with a reduction of markers reflecting bone formation [5–8]. An excess of bone resorption also has been documented by morphometric analysis of bone biopsies [9]. Finally, as far as we know, there has been just one study in the literature which evaluates bone mineral density (BMD) in patients with MGUS. We, in fact, have demonstrated osteoporotic values, according to the World Health Organization classification, in 26.2% of female patients [4].
Recent characterization of novel molecules, such as of the receptor activator of nuclear factor κB ligand (RANKL), osteoprotegerin (OPG), and macrophage inflammatory protein-1α (MIP-1α), has provided new insights into the pathophysiology of bone disease in these disorders. For example, the mean peripheral RANKL/OPG concentration ratio has been shown to be significantly higher in patients with multiple myeloma, and a similar result has been documented in a relatively small group of patients with MGUS [10, 11]. According to these results, we recently observed that in MGUS patients with vertebral fractures the mean RANKL/OPG ratio was significantly increased compared with mean values in MGUS patients without fractures [4].
These data considered as a whole suggest that patients affected by monoclonal gammopathy should be considered at risk of fracture; moreover, it appears that the pathophysiological mechanism of this increased risk could be ascribed to the raised bone turnover rate, probably due to an altered relationship of the RANKL/OPG balance at the bone marrow level. Therefore, it seems rational that these patients could benefit by the use of drugs that normalize the negative balance at the level of single remodeling units, thus decreasing the risk of fracture.
Bisphosphonates are among the drugs which have long been utilized in the treatment of osteoporotic patients [12–14]. They are not nowadays prescribed for MGUS patients, while they are recommended for patients affected by multiple myeloma [15]. The aim of our study was to evaluate the effect of alendronate treatment together with calcium supplements and cholecalciferol for a period of 18 months in a group of male and female osteoporotic MGUS patients.
Materials and Methods
Patients
In January 2002, we contacted by telephone 120 patients suffering from de novo MGUS diagnosed at the Hematology Institute of the University of Rome “La Sapienza” between 1980 and 2001. The diagnosis of MGUS was made according to standard criteria (briefly, serum protein electrophoresis showing a monoclonal protein concentration <30 g/L, plasma cell content <10% in the bone marrow examination, absence of renal failure, anemia, hypercalcemia, and lytic bone lesions when the X-ray skeletal examination was carried out). In each patient immunofixation was performed to determine the type of monoclonal protein. After being contacted, the patients came in the same year, from March to May, to our Mineral Metabolism Center. After informed consent was obtained from the participants, each patient underwent a general medical examination and biochemical routine tests to exclude possible causes of secondary osteoporosis. None of the patients enrolled had ever taken any treatment which could interfere with mineral metabolism (steroids, diuretics, thyroid hormones, anticonvulsant drugs, lithium, etc.). Other exclusion criteria were a previous diagnosis of involutional osteoporosis and treatment with active drugs affecting skeletal turnover (estrogens and progestins, bisphosphonates, fluorides, calcitonin, calcium, and vitamin D supplements). The study was approved by our local ethics committee, and patient confidentiality was protected. We finally enrolled 100 patients (65 postmenopausal women and 35 men); this group included 94 patients with immunoglobulin G (IgG), five patients with IgA, and one patient with IgM monoclonal serum protein.
Laboratory Methods
Each patient enrolled in the study underwent a morning fasting blood sample, utilizing red-stoppered Vacutainers (BD Diagnostics, Buccinasco, Italy; for the determination of serum protein electrophoresis, creatinine, main parameters of calcium/phosphorus metabolism, and markers of skeletal turnover) and one purple-stoppered Vacutainer for hemochrome measurement. Apart from the routine blood tests carried out the same day, the blood samples were immediately centrifuged, separated in aliquots, and frozen at −80°C until assayed. The assays were completed within 3 months from the time the blood samples were taken. Hematological parameters were determined using a hematology analyzer (Bayer ADVIA 120; Diamond Diagnostics, Holliston, MA). Serum protein electrophoresis and immunofixation were evaluated by the Hydrasys system (Sebia, Florence, Italy). Serum levels of total calcium (Ca), phosphorus (P), and creatinine (Cr) were measured by means of a multichannel analyzer (Autoanalyzer RA 500; Technicon, Tarrytown, NY). Ionized Ca was measured, immediately after sampling, by use of an ion-specific electrode (Nova 8; Nova Biochemical, Waltham, MA), as previously described [16]. Cr clearance (CrCl) was estimated by the Cockcroft and Gault formula [17], taking into consideration serum Cr (expressed in micromoles per liter), age (years), body weight (kilograms), and gender: estimated Cr clearance = [(140 – age) × weight × K]/serum Cr, the constant K being equal to 1.04 for women and 1.24 for men. Serum levels of calcidiol [25(OH)D] were determined by radioimmunoassay (DiaSorin, Stillwater, MN) as previously described [18]. Intra- and interassay coefficients of variation for the method were 8.1% and 10.2%, respectively. Circulating parathyroid hormone (PTH) levels were determined by an immunoradiometric assay that measures serum hormone levels using two affinity-purified polyclonal antibodies, one specific for the amino-terminal 1–34 portion of the PTH molecule and one specific for the 39–84 sequence of the hormone (N-tact PTHSP, DiaSorin). Intra- and interassay coefficients of variation, in our laboratory, were less than 3.0% and 5.5%, respectively [19]. Serum levels of C-terminal telopeptides of collagen type I (βCTX) were measured by enzyme-linked immunosorbent assay (Serum CrossLaps ELISA; Nordic Bioscience Diagnostics, Herlev, Denmark) [20]. Intra- and interassay coefficients of variation, in our laboratory, were less than 5.1% and 5.4%, respectively. Bone isoenzyme of alkaline phosphatase (BALP) was determined with an immunoenzymatic assay (Metra BAP EIA Kit; Quidel, San Diego, CA) [21]; intra- and interassay coefficients of variation were less than 5.6% and 7.8%, respectively. Serum osteocalcin (or bone Gla protein, BGP) was determined with an immunoradiometric assay (N-tact Osteo SP, DiaSorin) [22]; intra- and interassay coefficients of variation were less than 4.5% and 9.5%, respectively. Bone sialoprotein (BSP) was determined using a radioimmunoassay (Immunodiagnostik, Bensheim, Germany) described elsewhere [23]. Intra- and interassay coefficients of variation were less than 6% and 9%, respectively. Each patient underwent standardized lateral radiographs of the thoracic and lumbar spine, centred at T8 and L3, respectively, at a film focus distance of 105 cm. After visual inspection of these radiographs by two independent experienced observers, vertebral deformity was defined when anterior, middle, or posterior height loss was >20% with respect to the adjacent vertebra, according to Genant’s method [24]. BMD of the lumbar spine (L1–L4) in the anterior–posterior projection and of the hip (femoral neck and total hip) was measured in each patient with dual-energy X-ray absorptiometry (QDR 4500; Hologic, Waltham, MA). The precision errors of lumbar spine and total hip measurements were 1.3% and 1.7%, respectively.
Study Design
Following the first medical examination, those patients who satisfied our inclusion/exclusion criteria were divided into two groups according to the presence of vertebral osteoporotic fractures and/or a densitometric diagnosis of osteoporosis (in at least one of the two sites examined) along with World Health Organization criteria [25]. The first group of patients with fractures and/or osteoporotic densitometric values was treated with alendronate 70 mg a week per os 30 minutes before breakfast, calcium supplement (1,000 mg/day), and cholecalciferol (880 UI/day) per os for 18 months (group A). The second group of patients without osteoporotic fractures and normal or osteopenic densitometric values was treated with a calcium supplement (1,000 mg/day) and cholecalciferol (880 UI/day) per os for 18 months (group B). Every 6 months each patient underwent hematochemical tests including hemochrome, serum electrophoresis with monoclonal component evaluation, Cr, BALP, BGP, BSP, and βCTX. Each patient then, every 6 months, underwent a lumbar and femoral BMD measurement.
Statistical Analysis
The data are expressed as mean ± standard deviation (SD). Because the data distribution did not follow a gaussian curve, nonparametric tests were carried out. Variations between the various times considered were tested using the Friedman test and the Wilcoxon test for paired ranks. The groups were compared using the Mann-Whitney test for ranks. Results were considered significant at P < 0.05. Statistical elaborations were carried out using the SPSS program (release 10; SPSS, Inc., Chicago, IL).
Results
Eighty-three percent of patients contacted by telephone satisfied our established criteria and/or were willing to participate in the investigation. Therefore, we initially recruited 100 patients affected by MGUS (65 women and 35 men).
Main anthropometric, biochemical, and densitometric data together with the number and type of fractures in the female group have been previously reported [4]. Table 1 shows anthropometric, biochemical, and densitometric data in the male group, considered as a whole. In this group, morphometric X-ray examination of the spine showed that there were nine patients with at least one mild vertebral fracture and three with two vertebral fractures. One male patient who had a vertebral fracture also had a positive history of Colles fracture.
The initial group studied (65 females and 35 males) was further subdivided into two groups on the basis of the presence or absence of skeletal fragility, i.e., fractures and/or osteoporosis. Table 2 shows the composition of the two groups which resulted from this subdivision (each included 50 patients). The group with skeletal fragility underwent pharmacological treatment with alendronate, Ca, and cholecalciferol, as described in “Materials and Methods” (group A). The group without osteoporotic fractures and/or osteoporosis underwent only Ca and cholecalciferol supplementation (group B).
During the whole period of investigation (i.e., 18 months), eight patients in group A developed multiple myeloma and therefore were not able to continue the study. A further 12 patients included in group A did not want to take the drugs prescribed, for various reasons (four patients due to a lack of interest in the study, three patients due to moving to another city, one patient due to problems related to the medication prescribed, and another four patients due to personal reasons). However, nine of them, recalled at the end of the study, agreed to have BMD measurement at 18 months. Twenty-one patients from group B did not continue the entire follow-up mainly because of a lack of interest in undergoing periodic visits since they had normal densitometric values. Ten of these patients complained of gastric discomfort of various degrees after oral Ca intake; in the end, one of these patients was found positive for hepatitis C virus (HCV) and, therefore, decided to interrupt the study. However, 14 patients of this group agreed to have a BMD measurement at the end of the study. Statistical elaborations were carried out only for the patients who completed the entire study at 18 months (group A = 30 patients, 23 women and 7 men; group B = 29 patients, 17 women and 12 men) (Tables 3, 4). However, the results of the nine patients in group A and of the 14 patients in group B who had only the 18-month BMD measurement are also reported.
We found (according to the diagnostic criteria of bone mass measurement of the World Health Organization) that 46% of the patients in group A had osteoporotic densitometric values at the lumbar and/or hip level, 42% had osteopenic values, and 12% had normal values. In group B we found that 54% of the patients had osteopenic densitometric values at the lumbar and/or hip level and 46% of the patients had normal values at each site examined.
The two groups examined were not significantly different regarding mean age or mean time since initial MGUS diagnosis and were not different regarding mean values of hemoglobin, Cr, Cr clearance, and the monoclonal component (Table 3).
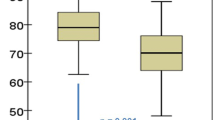
Figure 1 shows percentage changes from the basal values of BMD at the lumbar and hip sites in both group A with skeletal fragility taking alendronate therapy and group B taking only Ca and cholecalciferol supplements. After 18 months, the mean BMD of the lumbar spine and total femur had increased by 6.1 ± 1.1% (mean ± standard error [SE]) and 1.5 ± 0.7%, respectively, in osteoporotic MGUS patients treated with alendronate; mean changes of lumbar BMD observed at 6, 12, and 18 months were significantly different from mean basal values. In the nine patients of group A not taking alendronate, BMD values of the lumbar spine and total femur decreased by 1.6 ± 0.7% (P ≤ 0.001) and 1.3 ± 0.4% (P ≤ 0.01), respectively. In patients of group B, BMD increased by 1.2 ± 0.7% at the lumbar spine and decreased by 1.2 ± 0.5% at the total femur. Corresponding figures of those patients in the same group not taking calcium and vitamin D supplementation were −0.1% and −1.2%, respectively.
Figure 2 illustrates the percent changes from baseline of the biochemical markers of bone turnover. After starting therapy with alendronate, there was a marked decrease in mean serum ßCTX, which persisted throughout the treatment period in osteoporotic MGUS patients; smaller mean decreases were observed concerning the other markers investigated. We also found a significant inverse correlation between changes of lumbar spine BMD and serum ßCTX (r = −0.37, P = 0.03). In MGUS patients without osteoporosis, we observed significant decreases of bone remodeling markers (the only exception being mean BSP) following therapy with cholecalciferol and Ca; however, these reductions were consistently lower than those observed in group A, even though there was no statistically significant difference in the mean reduction of BSP values at the end of the study. At 18 months, the absolute percent difference between the groups was −23.2 (P ≤ 0.0l) for serum BALP, −23.6 for serum BGP (P ≤ 0.0l), −35.1 for serum ßCTX (P ≤ 0.001) and − 0.47 (P = nonsignificant) for serum BSP.
Finally, we did not find any significant mean change of the monoclonal component value during the observation period in both groups.
Discussion
It is nowadays clearly demonstrated that patients affected by MGUS should be considered at increased risk of fracture, especially at the axial level [2–4]. Similar results were found in the population we studied; indeed, we observed a prevalence of axial fractures of approximately 52% in the female group and 34% in the male group. These percentages are particularly elevated when compared with those in the general population [26, 27]; they also appear to be high in relation to studies carried out with the same definition criteria of vertebral fractures [28, 29]. In this context, it is important to underline that grade I vertebral deformity according to the Genant method [24] should be considered an early sign of skeletal fragility [30] and, consequently, should be treated. Surprisingly, even though skeletal fragility is well documented, there are no data in the literature about interventional studies for primary and/or secondary prevention in this disease.
In previous studies carried out by our group [4] and by other investigators [5, 6, 9, 10], it has been shown that MGUS patients are characterized by an alteration in skeletal remodeling, with a prevalence of bone resorption over formation. Therefore, one of the ways by which bone loss could be prevented is by giving a drug that inhibits bone resorption. Therefore, we decided to carry out an 18-month open label pharmacological study by administering a bisphosphonate used worldwide for the treatment of osteoporosis, such as alendronate.
Following alendronate therapy in osteoporotic patients affected by MGUS, we found a significant increase in lumbar BMD compared with basal values after only 6 months of treatment. This trend was observed throughout the period of observation (i.e., 18 months), reaching at the end a mean percentage increase of 6.1% (Fig. 1). It is important to note that the latter figure is similar to the value which may be derived after 18 months of treatment with alendronate in studies carried out for a longer period of time in postmenopausal women with osteoporosis, with or without vertebral fractures [13, 31, 32]. Moreover, the same increase in lumbar BMD was found in another study carried out in a male population treated with alendronate for 18 months [33]. Considering the 1.6% reduction of BMD of MGUS osteoporotic patients without treatment observed at 18 months, this results in an absolute difference between the two groups of 7.7% (P ≤ 0.001). Concerning the hip site, we did not find a statistically significant increase in the osteoporotic group during the whole period of observation; however, the absolute difference with respect to those not taking alendronate was 2.8% (P ≤ 0.01). The lower increase in densitometric values at the hip site compared with the lumbar site after pharmacological treatment with bisphosphonates is already known in the literature. Nonetheless, due to the fact that, from a clinical point of view, the fracture risk in patients affected by MGUS is higher at the axial level compared to the hip level [2, 34], the therapeutic effect at the hip level should be considered less relevant.
The changes of bone turnover markers after alendronate treatment are in line with the well-known initial inhibition of bone resorption, following which the reduction of formation markers is observed. The same changes have been documented in the literature after treatment with alendronate of osteoporotic patients of both sexes [33, 35]. A minimal reduction in the markers of bone turnover was also documented in patients treated with Ca and cholecalciferol (group B) with the exception of BALP. The slight reduction in bone turnover with Ca and cholecalciferol administration might be related to the beneficial effect of correct supplementation, often not optimized even in our geographical area [36, 37]. However, it should be noted that, with the exception of BSP values at 18 months, the mean reduction observed for all the markers in group B was always significantly lower than that documented in the group treated with antiresorptive therapy. It is interesting to note the time course of changes of BSP, a noncollagenic bone matrix protein physiologically involved in the adhesion of osteoclasts to the matrix itself. Indeed, the results obtained following the first 12 months of observation could be considered similar to those obtained for the other markers. However, at the end of the treatment period, a reduction similar to those obtained in patients treated with alendronate was shown even in the patients of group B. We do not have a clear interpretation for this result; a possible explanation could be a different sensitivity of this in respect to other markers.
Regarding the mechanism of action of alendronate, one cannot also exclude a direct effect on the RANKL/OPG system; it is worth emphasizing that this effect has been observed also in postmenopausal osteoporotic patients treated with the same drug [38]. This action could counteract the imbalance in favor of osteoclastogenesis of the RANKL/OPG ratio documented in MGUS patients. Other drugs that act directly at this level could be used in the treatment of these hematological diseases [39]. Finally, the finding of no significant change of the monoclonal component in MGUS patients given alendronate is in accordance with previous studies carried out in myeloma patients showing that other bisphosphonates (i.e., pamidronate and zoledronic acid) are unable to modify this component [40].
This study has some limitations, mainly because of the lack of a real control group (longitudinally followed for the entire observation period) and the lack of morphometric evaluation of vertebral fractures at 18 months. Regarding the first point, our ethical committee found supplementing patients who had a vertebral fracture with only Ca and vitamin D unacceptable. This is due to the fact that there is no evidence-based proof of secondary prevention of fractures with only this regimen. However, we partly overcame this problem by measuring BMD of those patients not taking the drug at 18 months. Regarding the second point, no clinical fracture was documented in the sample studied, even though this is biased by the known evidence that two-thirds of vertebral fractures are asymptomatic [26]. However, we can reasonably infer that, as is well documented in other groups of patients treated with alendronate, both the increase in bone density and the reduction in bone turnover are expected to lead to a significant reduction in fracture risk even in patients affected by MGUS [41, 42].
However, it must be noted that this study presents a number of strengths. In fact, this is the first time that the utility of pharmacological treatment for increasing BMD at a lumbar site in patients affected by MGUS has been shown. The lumbar spine is, in fact, the site of the most frequent fracture occurrence in patients with MGUS. Also, in contrast to previous studies, both sexes were studied, thus allowing translation of the results to both males and females. Finally, we have shown that simple supplementation with Ca and vitamin D positively influences bone turnover in nonosteoporotic patients with MGUS, thus preventing bone loss.
In conclusion, our study represents a basis for better treatment of osteoporotic patients suffering from MGUS, even though further studies with a larger sample and with both densitometric and morphometric end points are needed.
References
Kyle RA, Therneau TR, Rajkumar SV, Karson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ III (2006) Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354:1362–1369
Melton LJ III, Rajkumar SV, Khosla S, Achenbach SJ, Oberg AL, Kyle RA (2004) Fracture risk in monoclonal gammopathy of undetermined significance. J Bone Miner Res 19:25–30
Gregersen H, Jensen P, Gislum M, Jorgensen B, Sorensen HT, Norgaard M (2006) Fracture risk in patients with monoclonal gammopathy of undetermined significance. Br J Haematol 135:62–67
Pepe J, Petrucci MT, Nofroni I, Fassino V, Diacinti D, Romagnoli E, Minisola S (2006) Lumbar bone mineral density as the major factor determining increased prevalence of vertebral fractures in monoclonal gammopathy of undetermined significance. Br J Haematol 134:485–490
Pecherstorfer M, Seibel MJ, Woitge HW, Horn E, Schuster J, Neuda J, Sagaster P, Kohn H, Bayer P, Thiebaud D, Ludwig H (1997) Bone resorption in multiple myeloma and in monoclonal gammopathy of undetermined significance: quantification by urinary pyridinium cross-links of collagen. Blood 90:3743–3750
Vejlgaard T, Abildgaard N, Jans H, Nielsen JL, Heickendorff L (1997) Abnormal bone turnover in monoclonal gammopathy of undetermined significance: analysis of type I collagene telopeptide, osteocalcin, bone-specific alkaline phosphatase and propeptides of type I and type III procollagenes. Eur J Haematol 58:104–108
Diamond T, Levy S, Smith A, Day P, Manoharan A (2001) Non-invasive markers of bone turnover and plasma cytokines differ in osteoporotic patients with multiple myeloma and monoclonal gammopathies of undetermined significance. Intern Med J 31:272–278
Jakob C, Zavrski I, Heider U, Brux B, Eucker J, Langelotz C, Sinha P, Possinger K, Sezer O (2002) Bone resorption parameters carboxy-terminal telopeptide of type I collagen (ICTP), amino-terminal collagen type I telopeptide (NTx), and deoxypyridinoline (DpD) in MGUS and multiple myeloma. Eur J Haematol 69:37–42
Bataille R, Chappard D, Basle MF (1996) Quantifiable excess of bone resorption in monoclonal gammopathy is an early symptom of malignancy: a prospective study of 87 bone biopsies. Blood 87:4762–4769
Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, Viniou N, Yataganas X, Goldman JM, Rahemtulla A (2003) Soluble receptor activator of nuclear factor-kappa B ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood 102:1064–1069
Politou M, Terpos E, Anagnostopoulos A, Szydlo R, Laffan M, Layton M, Apperley JF, Dimopoulos MA, Rahemtulla A (2004) Role of receptor activator of nuclear factor-kappa B ligand (RANKL), osteoprotegerin and macrophage protein 1-alpha (MIP-1α) in monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol 126:686–689
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY (2001) Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med 344:330–340
Berenson JR, Hillner BE, Kyle RA, Anderson K, Lipton A, Yee GC, Biermann JS (2002) American Society of Clinical Oncology Bisphosphonates Expert Panel. American Society of Clinical Oncology clinical practice guidelines: the role of bisphosphonates in multiple myeloma. J Clin Oncol 20:3719–3736
Minisola S, Pacitti MT, Scarda A, Rosso R, Romagnoli E, Carnevale V, Scarnecchia L, Mazzuoli GF (1993) Serum ionized calcium, parathyroid hormone and related variables: effects of age and sex. Bone Miner 23:183–193
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL (1993) Determination of vitamin D status by radioimmunoassay with 125I labeled tracer. Clin Chem 39:529–533
Pepe J, Romagnoli E, Nofroni I, Pacitti MT, De Geronimo S, Letizia C, Tonnarini G, Scarpiello A, D’Erasmo E, Minisola S (2005) Vitamin D status as the major factor determining the circulating levels of parathyroid hormone: a study in normal subjects. Osteoporos Int 16:805–812
Rosenquist C, Fledelius C, Christgau S, Pedersen BJ, Bonde M, Qvist P, Christiansen C (1998) Serum CrossLaps one step ELISA. First application of monoclonal antibodies for measurement of bone-related degradation products from C-terminal telopeptides of type I collagen. Clin Chem 44:2281–2289
Gomez B Jr, Ardakani S, Ju J, Jenkins D, Cerelli MJ, Daniloff GY, Kung VT (1995) Monoclonal antibody assay for measuring bone specific alkaline phosphatase activity in serum. Clin Chem 41:1560–1566
Minisola S, Pacitti MT, Romagnoli E, Rosso R, Carnevale V, Caravella P, Scillitani A, Dicembrino F (1999) Clinical validation of a new immunoradiometric assay for intact human osteocalcin. Calcif Tissue Int 64:365–369
Karmatschek M, Maier I, Seibel MJ, Woitge HW, Ziegler R, Armbruster FP (1997) Improved purification of human bone sialoprotein and development of a homologous radioimmunoassay. Clin Chem 43:2076–2082
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 9:1137–1148
Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141
Cummings SR, Melton LJ III (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767
O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ (1996) The prevalence of vertebral deformity in European men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 11:1010–1018
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis the Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11:984–996
Vogt TM, Ross PD, Palermo L, Musliner T, Genant HK, Black D, Thompson DE (2000) Vertebral fracture prevalence among women screened for the Fracture Intervention Trial and a simple clinical tool to screen for undiagnosed vertebral fractures. Fracture Intervention Trial Research Group. Mayo Clin Proc 75:888–896
Minisola S, Pepe J, Romagnoli E (2007) Monoclonal gammopathy of undetermined significance. N Engl J Med 356:2223–2224
Pols HA, Felsenberg D, Hanley DA, Stepan J, Mũnoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K, Yates AJ, Stych B (1999) Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int 9:461–468
Liberman UA, Weiss SR, Bröll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M, Seeman E, Recker RR, Capizzi T, Santora AC, Lombardi A, Shah RV, Hirsh LJ, Karpf DB (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The alendronate phase III osteoporosis treatment study group. N Engl J Med 333:1437–1443
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Dhodapkar MV, Weinstein R, Tricot G, Jagannath S, Parfitt AM, Manolagas SC, Barlogie B (1998) Biologic and therapeutic determinants of bone mineral density in multiple myeloma. Leuk Lymphoma 32:121–127
Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD (1994) Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab 79:1693–1700
Romagnoli E, Caravella P, Scarnecchia L, Martinez P, Minisola S (1999) Hypovitaminosis D in an Italian population of healthy subjects and hospitalized patients. Br J Nutr 81:133–137
van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA (1995) Serum vitamin D concentrations among elderly people in Europe. Lancet 346:207–210
Dobnig H, Hofbauer LC, Viereck V, Obermayer-Pietsch B, Fahrleitner-Pammer A (2006) Changes in the RANK ligand/osteoprotegerin system are correlated to changes in bone mineral density in bisphosphonate-treated osteoporotic patients. Osteoporos Int 17:693–703
Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ (2006) A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res 15:1221–1228
Musto P, Falcone A, Sanpaolo G, Bodenizza C, Cascavilla N, Melillo L, Scalzulli PR, Dell’Olio M, La Sala A, Mantuano S, Nobile M, Carella AM (2003) Pamidronate reduces skeletal events but does not improve progression-free survival in early-stage untreated myeloma: results of a randomized trial. Leuk Lymphoma 44:1545–1548
Hochberg MC, Ross PD, Black D, Cummings SR, Genant HK, Nevitt MC, Barrett-Connor E, Musliner T, Thompson D (1999) Larger increases in bone mineral density during alendronate therapy are associated with a lower risk of new vertebral fractures in women with postmenopausal osteoporosis. Fracture Intervention Trial Research Group. Arthritis Rheum 42:1246–1254
Wasnich RD, Miller PD (2000) Antifracture efficacy of antiresorptive agents are related to changes in bone density. J Clin Endocrinol Metab 85:231–236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pepe, J., Petrucci, M.T., Mascia, M.L. et al. The Effects of Alendronate Treatment in Osteoporotic Patients Affected by Monoclonal Gammopathy of Undetermined Significance. Calcif Tissue Int 82, 418–426 (2008). https://doi.org/10.1007/s00223-008-9145-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9145-2