Abstract
Purpose
Peptide receptor radionuclide therapy (PRRT) is used in tumours expressing type 2 somatostatin receptors (sst2), mainly neuroendocrine. The aim of this prospective phase I-II study was to evaluate the toxicity and efficacy of 177Lu-DOTATATE in multiple cycles.
Methods
Fifty-one consecutive patients with unresectable/metastatic sst2-positive tumours, divided into two groups, received escalating activities (3.7–5.18 GBq/cycle, group 1; 5.18–7.4 GBq/cycle, group 2) of 177Lu-DOTATATE. Cumulative activities ranged from 3.7 to 29.2 GBq (median 26.4 GBq in median 6 cycles, group 1, 21 patients) and 5.55 to 28.9 GBq (median 25.2 GBq in 4 cycles, group 2, 30 patients), based on dosimetry.
Results
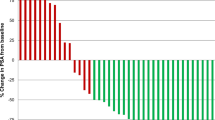
No major acute or delayed renal or haematological toxicity occurred (one grade 3 leukopenia and thrombocytopenia). Cumulative renal absorbed doses were 8–37 Gy (9–41 Gy bioeffective doses). A median decrease of creatinine clearance of 21.7% 6 months after PRRT, 23.9% after 1 year and 27.6% after 2 years was observed. Higher losses (>20%) occurred in patients with risk factors for renal toxicity, particularly hypertension and diabetes. Cumulative bone marrow doses were <1.5 Gy. Blood elements showed a progressive mild drop during cycles and recovered during follow-up (median 30 months). Thirty-nine patients were progressive at enrolment. Partial and complete responses occurred in 15 of 46 (32.6%) assessable patients. The median time to progression was 36 months. Overall survival was 68% at 36 months. Non-responders and patients with extensive tumour involvement had lower survival.
Conclusion
177Lu-DOTATATE was well tolerated up to 29 GBq cumulative activity (up to 7.4 GBq/cycle). The maximum tolerated dose/cycle was not reached. However, considering the individual bone marrow function and the presence of risk factors for kidney toxicity, it seems safer to divide cumulative activities into lower activity cycles.





Similar content being viewed by others
References
Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9(1):61–72.
Bodei L, Ferone D, Grana CM, Cremonesi M, Signore A, Dierkxs RA, et al. Peptide receptor therapies in neuroendocrine tumors. J Endocrinol Invest 2009;32(4):360–9.
Kwekkeboom DJ, Kam BL, van Essen M, Teunissen JJ, van Eijck CH, Valkema R, et al. Somatostatin-receptor-based imaging and therapy of gastroenteropancreatic neuroendocrine tumors. Endocr Relat Cancer 2010;17(1):R53–73.
Bartolomei M, Bodei L, De Cicco C, Grana CM, Cremonesi M, Botteri E, et al. Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging 2009;36(9):1407–16.
Cecchin D, Schiavi F, Fanti S, Favero M, Manara R, Fassina A, et al. Peptide receptor radionuclide therapy in a case of multiple spinal canal and cranial paragangliomas. J Clin Oncol 2011;29(7):e171–4.
Virgolini I, Ambrosini V, Bomanji JB, Baum RP, Fanti S, Gabriel M, et al. Procedure guidelines for PET/CT tumour imaging with 68Ga-DOTA-conjugated peptides: 68Ga-DOTA-TOC, 68Ga-DOTA-NOC, 68Ga-DOTA-TATE. Eur J Nucl Med Mol Imaging 2010;37(10):2004–10.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48(4):508–18.
Ambrosini V, Campana D, Bodei L, Nanni C, Castellucci P, Allegri V, et al. 68Ga-DOTANOC PET/CT clinical impact in patients with neuroendocrine tumors. J Nucl Med 2010;51(5):669–73.
Cremonesi M, Botta F, Di Dia A, Ferrari M, Bodei L, De Cicco C, et al. Dosimetry for treatment with radiolabelled somatostatin analogues. A review. Q J Nucl Med Mol Imaging 2010;54(1):37–51.
Breeman WAP, de Blois E, Bakker WH, Krenning EP. Radiolabeling DOTA-peptides with 90Y and 177Lu to a high specific activity. In: Chinol M, Paganelli G, editors. Radionuclide peptide cancer therapy. New York: Taylor & Francis Group; 2006. p. 119–26.
Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in peptide radionuclide receptor therapy: a review. J Nucl Med 2006;47(9):1467–75.
Dale R. Use of the linear-quadratic radiobiological model for quantifying kidney response in targeted radiotherapy. Cancer Biother Radiopharm 2004;19(3):363–70.
Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med 2005;46 Suppl 1:99S–106.
Wessels BW, Konijnenberg MW, Dale RG, Breitz HB, Cremonesi M, Meredith RF, et al. MIRD pamphlet No. 20: the effect of model assumptions on kidney dosimetry and response—implications for radionuclide therapy. J Nucl Med 2008;49(11):1884–99.
Common Terminology Criteria for Adverse Events v3.0 (CTCAE, http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
Bryant J, Day R. Incorporating toxicity considerations into the design of two stage phase II clinical trials. Biometrics 1995;51:1372–81.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16(1):31–41.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23:2754–62.
Kwekkeboom DJ, de Herder WW, Kam BL, van Eijck CH, van Essen M, Kooij PP, et al. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol 2008;26:2124–30.
Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 2008;35(10):1847–56.
Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352:1011–23.
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0),Tyr(3)-octreotate. J Nucl Med 2005;46 Suppl 1:83S–91.
Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol 2009;27(28):4656–63.
Willers H, Held KD. Introduction to clinical radiation biology. Hematol Oncol Clin North Am 2006;20(1):1–24.
Teunissen JJ, Kwekkeboom DJ, Krenning EP. Quality of life in patients with gastroenteropancreatic tumors treated with [177Lu-DOTA0,Tyr3]octreotate. J Clin Oncol 2004;22(13):2724–9.
Davì MV, Bodei L, Ferdeghini M, Falconi M, Testoni M, Paganelli G, et al. Multidisciplinary approach including receptor radionuclide therapy with 90Y-DOTATOC ([90Y-DOTA0, Tyr3]-octreotide) and 177Lu-DOTATATE ([177Lu-DOTA0, Tyr3]-octreotate) in ectopic Cushing syndrome from a metastatic gastrinoma: a promising proposal. Endocr Pract 2008;14(2):213–8.
Acknowledgments
The authors thank Mrs. Deborah Console for the editing of this manuscript.
Conflicts of interest
LB and GP declare one-occasion consultancy with Advanced Accelerator Applications during the preliminary phase of product development of 177Lu-DOTATATE in Italy. All other authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bodei, L., Cremonesi, M., Grana, C.M. et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging 38, 2125–2135 (2011). https://doi.org/10.1007/s00259-011-1902-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-011-1902-1