Abstract
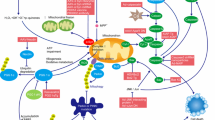
Gene therapy represents a potentially useful additional technique to ameliorate the motor symptoms of Parkinson’s disease (PD), and the motor complications of its treatment. The neurodegenerative process itself, as well as the non-motor symptoms of PD, both remain less amenable to most of the current gene therapy approaches. This review presents an overview of the four gene therapies in phase I/II clinical trials, outlines some of the challenges they face, and proposes additional alternative strategies that might improve the clinical prospects of gene therapy for PD. In so doing, we hope to highlight the issue of the current absence of effective treatment for non-motor symptoms of PD and the potential of further candidate targets for gene therapy intervention that might improve upon this, for both specific individuals with genetic forms of PD as well as “sporadic” PD patients.

Similar content being viewed by others
References
Quinn N (1995) Drug treatment of Parkinson’s disease. BMJ 310(6979):575–579
Bezard E, Brotchie JM, Gross CE (2001) Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci 2(8):577–588
Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260(5111):1130–1132
Stromberg I, Bjorklund L, Johansson M, Tomac A, Collins F, Olson L, Hoffer B, Humpel C (1993) Glial cell line-derived neurotrophic factor is expressed in the developing but not adult striatum and stimulates developing dopamine neurons in vivo. Exp Neurol 124(2):401–412
Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA (1996) Functional recovery in parkinsonian monkeys treated with GDNF. Nature 380(6571):252–255
Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LF, Gerhardt GA (1994) Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett 182(1):107–111
Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, Olson L (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373(6512):335–339
Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 290(5492):767–773
Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9(5):589–595
Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, Walton A, Wagner R, Young AB (2007) Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg 106(4):614–620
Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59(3):459–466
Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER Jr, Lozano AM, Penn RD, Simpson RK Jr, Stacy M, Wooten GF (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60(1):69–73
Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM Jr (1997) Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA 94(13):7018–7023
Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson E Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS (1998) Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J Neurosci 18(13):4929–4937
Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A (1999) Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. Eur J Neurosci 11(5):1554–1566
Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J 3rd, Gasmi M, Bartus RT (2006) Delivery of neurturin by AAV2 (cere-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60(6):706–715
Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT (2007) AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of cere-120 for Parkinson’s disease. Neurobiol Dis 27(1):67–76
Marks WJ Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT (2008) Safety and tolerability of intraputaminal delivery of cere-120 (adeno-associated virus serotype-2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol 7(5):400–408
Ceregene (2009) Ceregene announces clinical data from phase 2 clinical trial of cere-120 for Parkinson’s disease. http://ceregene.com/press_112608.asp. Accessed November 22 2009
Ceregene (2009) Ceregene announces clinical data from phase 2 clinical trial of cere-120 for Parkinson’s disease—longer term follow-up indicates modest efficacy in primary and related endpoints. http://ceregene.com/press_052709.asp. Accessed November 22 2009
Ceregene (2009) Ceregene receives additional grant from Michael J. Fox foundation to expand lond-term testing of cere-120 patiens. http://www.ceregene.com/press_080509.asp. Accessed 20 September 2010
Ceregene (2010) Ceregene has initiated a new phase1/2 trial of cere-120 for Parkinson’s disease. http://www.ceregene.com/press-042810.asp. Accessed 20 September 2010
Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, Cunningham J (2006) Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-HAADC. Mol Ther 14(4):564–570
Nagatsu T, Yamaguchi T, Kato T, Sugimoto T, Matsuura S, Akino M, Nagatsu I, Iizuka R, Narabayashi H (1981) Biopterin in human brain and urine from controls and parkinsonian patients: application of a new radioimmunoassay. Clin Chim Acta 109(3):305–311
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA, VanBrocklin HF, Wright JF, Bankiewicz KS, Aminoff MJ (2009) Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73(20):1662–1669
Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ (2008) Results from a phase I safety trial of HAADC gene therapy for Parkinson disease. Neurology 70(21):1980–1983
Nagatsu T, Sawada M (2007) Biochemistry of postmortem brains in Parkinson’s disease: historical overview and future prospects. J Neural Transm 2007(Suppl 72):113–120
Muramatsu S, Wang L, Ikeguchi K, Fujimoto K, Nakano I, Ozawa K (2002) Recombinant adeno-associated viral vectors bring gene therapy for Parkinson’s disease closer to reality. J Neurol 249(Suppl 2):II36–II40
Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto KI, Fan DS, Ogawa M, Mizukami H, Urabe M, Kume A, Nagatsu I, Urano F, Suzuki T, Ichinose H, Nagatsu T, Monahan J, Nakano I, Ozawa K (2000) Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-l-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Hum Gene Ther 11(11):1509–1519
Levine RA, Miller LP, Lovenberg W (1981) Tetrahydrobiopterin in striatum: localization in dopamine nerve terminals and role in catecholamine synthesis. Science 214(4523):919–921
Azzouz M, Martin-Rendon E, Barber RD, Mitrophanous KA, Carter EE, Rohll JB, Kingsman SM, Kingsman AJ, Mazarakis ND (2002) Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic-l-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. J Neurosci 22(23):10302–10312
Jarraya B, Boulet S, Ralph GS, Jan C, Bonvento G, Azzouz M, Miskin JE, Shin M, Delzescaux T, Drouot X, Herard AS, Day DM, Brouillet E, Kingsman SM, Hantraye P, Mitrophanous KA, Mazarakis ND, Palfi S (2009) Dopamine gene therapy for Parkinson’s disease in a nonhuman primate without associated dyskinesia. Sci Transl Med 1(2):2ra4
OxfordBioMedica (2010) Oxford biomedica announces two-year phase I/II results of prosavin in Parkinson’s disease. http://www.oxfordbiomedica.co.uk/page.asp?pageid=59&newsid=259. Accessed 20 September 2010
Hammond C, Bergman H, Brown P (2007) Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30(7):357–364
Luo J, Kaplitt MG, Fitzsimons HL, Zuzga DS, Liu Y, Oshinsky ML, During MJ (2002) Subthalamic gad gene therapy in a Parkinson’s disease rat model. Science 298(5592):425–429
Lee B, Lee H, Nam YR, Oh JH, Cho YH, Chang JW (2005) Enhanced expression of glutamate decarboxylase 65 improves symptoms of rat parkinsonian models. Gene Ther 12(15):1215–1222
Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, Moirano J, Fitzsimons H, Roitberg BZ, Tuccar E, Roberts A, Kaplitt MG, Eidelberg D (2007) Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. J Cereb Blood Flow Metab 27(3):501–509
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ (2007) Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne gad gene for Parkinson’s disease: an open label, phase I trial. Lancet 369(9579):2097–2105
Bjorklund A, Bjorklund T, Kirik D (2009) Gene therapy for dopamine replacement in Parkinson’s disease. Sci Transl Med 1(2):2ps2
Bjorklund T, Kirik D (2009) Scientific rationale for the development of gene therapy strategies for Parkinson’s disease. Biochim Biophys Acta 1792(7):703–713
Hastings TG, Lewis DA, Zigmond MJ (1996) Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA 93(5):1956–1961
Chen MK, Kuwabara H, Zhou Y, Adams RJ, Brasic JR, McGlothan JL, Verina T, Burton NC, Alexander M, Kumar A, Wong DF, Guilarte TR (2008) VMAT2 and dopamine neuron loss in a primate model of Parkinson’s disease. J Neurochem 105(1):78–90
Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5(3):235–245
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Dickson DW, Fujishiro H, Orr C, DelleDonne A, Josephs KA, Frigerio R, Burnett M, Parisi JE, Klos KJ, Ahlskog JE (2009) Neuropathology of non-motor features of Parkinson disease. Parkinsonism Relat Disord 15(Suppl 3):S1–S5
Levy R, Dubois B (2006) Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 16(7):916–928
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128(Pt 6):1314–1322
Owen AM, Sahakian BJ, Semple J, Polkey CE, Robbins TW (1995) Visuo-spatial short-term recognition memory and learning after temporal lobe excisions, frontal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia 33(1):1–24
Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, Baudrexel S, Diederich NJ, Heiss WD, Hilker R (2010) Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 74(11):885–892
Whitworth AJ, Pallanck LJ (2009) The PINK1/parkin pathway: a mitochondrial quality control system? J Bioenerg Biomembr 41(6):499–503
Lo Bianco C, Schneider BL, Bauer M, Sajadi A, Brice A, Iwatsubo T, Aebischer P (2004) Lentiviral vector delivery of parkin prevents dopaminergic degeneration in an alpha-synuclein rat model of Parkinson’s disease. Proc Natl Acad Sci USA 101(50):17510–17515
Yamada M, Mizuno Y, Mochizuki H (2005) Parkin gene therapy for alpha-synucleinopathy: a rat model of Parkinson’s disease. Hum Gene Ther 16(2):262–270
Yasuda T, Miyachi S, Kitagawa R, Wada K, Nihira T, Ren YR, Hirai Y, Ageyama N, Terao K, Shimada T, Takada M, Mizuno Y, Mochizuki H (2007) Neuronal specificity of alpha-synuclein toxicity and effect of parkin co-expression in primates. Neuroscience 144(2):743–753
Mochizuki H (2009) Parkin gene therapy. Parkinsonism Relat Disord 15(Suppl 1):S43–S45
Zhou C, Huang Y, Przedborski S (2008) Oxidative stress in Parkinson’s disease: a mechanism of pathogenic and therapeutic significance. Ann NY Acad Sci 1147:93–104
Bueler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol 218(2):235–246
Barber-Singh J, Seo BB, Nakamaru-Ogiso E, Lau YS, Matsuno-Yagi A, Yagi T (2009) Neuroprotective effect of long-term NDI1 gene expression in a chronic mouse model of Parkinson disorder. Rejuvenation Res 12(4):259–267
Santosh PS, Arora N, Sarma P, Pal-Bhadra M, Bhadra U (2009) Interaction map and selection of microRNA targets in Parkinson’s disease-related genes. J Biomed Biotechnol 2009:363145
Tain LS, Mortiboys H, Tao RN, Ziviani E, Bandmann O, Whitworth AJ (2009) Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat Neurosci 12(9):1129–1135
Imai Y, Gehrke S, Wang HQ, Takahashi R, Hasegawa K, Oota E, Lu B (2008) Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J 27(18):2432–2443
Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, Wilson JM, Debyser Z, Baekelandt V (2007) Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther 18(3):195–206
McFarland NR, Lee JS, Hyman BT, McLean PJ (2009) Comparison of transduction efficiency of recombinant AAV serotypes 1, 2, 5, and 8 in the rat nigrostriatal system. J Neurochem 109(3):838–845
Ciron C, Cressant A, Roux F, Raoul S, Cherel Y, Hantraye P, Deglon N, Schwartz B, Barkats M, Heard JM, Tardieu M, Moullier P, Colle MA (2009) Human alpha-iduronidase gene transfer mediated by adeno-associated virus types 1, 2, and 5 in the brain of nonhuman primates: vector diffusion and biodistribution. Hum Gene Ther 20(4):350–360
Markakis EA, Vives KP, Bober J, Leichtle S, Leranth C, Beecham J, Elsworth JD, Roth RH, Samulski RJ, Redmond DE Jr (2010) Comparative transduction efficiency of AAV vector serotypes 1–6 in the substantia nigra and striatum of the primate brain. Mol Ther 18(3):588–593
Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM (2009) Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord 15(3):196–204
Burn D, Emre M, McKeith I, De Deyn PP, Aarsland D, Hsu C, Lane R (2006) Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord 21(11):1899–1907
Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E (2009) Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8(7):613–618
Kennington E (2009) Gene therapy delivers an alternative approach to Alzheimer’s disease. Nat Rev Drug Discov 8(4):275
Chaudhuri KR, Schapira AH (2009) Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8(5):464–474
Jasinska-Myga B, Putzke JD, Wider C, Wszolek ZK, Uitti RJ (2010) Depression in Parkinson’s disease. Can J Neurol Sci 37(1):61–66
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289(1–2):18–22
Muzerengi S, Contrafatto D, Chaudhuri KR (2007) Non-motor symptoms: identification and management. Parkinsonism Relat Disord 13(Suppl 3):S450–S456
Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R (2008) Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev 2008(2):CD006564
Fenelon G, Mahieux F, Huon R, Ziegler M (2000) Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain 123(Pt 4):733–745
Kumar S, Bhatia M, Behari M (2002) Sleep disorders in Parkinson’s disease. Mov Disord 17(4):775–781
Mitra T, Chaudhuri KR (2009) Sleep dysfunction and role of dysautonomia in Parkinson’s disease. Parkinsonism Relat Disord 15(Suppl 3):S93–S95
Comella CL (2008) Sleep disorders in Parkinson’s disease. Curr Treat Options Neurol 10(3):215–221
Lee JE, Kim KS, Shin HW, Sohn YH (2010) Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat Disord 16(2):105–108
Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, Carrier J, Montplaisir J (2002) REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology 59(4):585–589
Onofrj M, Thomas A, D’ Andreamatteo G, Iacono D, Luciano AL, Di Rollo A, Di Mascio R, Ballone E, Di Iorio A (2002) Incidence of RBD and hallucination in patients affected by Parkinson’s disease: 8-year follow-up. Neurol Sci 23(2):91–94
Arnulf I, Leu S, Oudiette D (2008) Abnormal sleep and sleepiness in Parkinson’s disease. Curr Opin Neurol 21(4):472–477
Lees AJ, Blackburn NA, Campbell VL (1988) The nighttime problems of Parkinson’s disease. Clin Neuropharmacol 11(6):512–519
Sakakibara R, Uchiyama T, Yamanishi T, Shirai K, Hattori T (2008) Bladder and bowel dysfunction in Parkinson’s disease. J Neural Transm 115(3):443–460
Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L (2006) Prevalence of bladder dysfunction in Parkinson’s disease. Neurourol Urodyn 25(2):116–122
Park A, Stacy M (2009) Non-motor symptoms in Parkinson’s disease. J Neurol 256(Suppl 3):293–298
Magerkurth C, Schnitzer R, Braune S (2005) Symptoms of autonomic failure in Parkinson’s disease: prevalence and impact on daily life. Clin Auton Res 15(2):76–82
Sakakibara R, Uchiyama T, Yamanishi T, Kishi M (2010) Genitourinary dysfunction in Parkinson’s disease. Mov Disord 25(1):2–12
Papatsoris AG, Deliveliotis C, Singer C, Papapetropoulos S (2006) Erectile dysfunction in Parkinson’s disease. Urology 67(3):447–451
Chou KL, Evatt M, Hinson V, Kompoliti K (2007) Sialorrhea in Parkinson’s disease: a review. Mov Disord 22(16):2306–2313
Kalf JG, de Swart BJ, Borm GF, Bloem BR, Munneke M (2009) Prevalence and definition of drooling in Parkinson’s disease: a systematic review. J Neurol 256(9):1391–1396
Pfeiffer RF (2003) Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2(2):107–116
Edwards LL, Pfeiffer RF, Quigley EM, Hofman R, Balluff M (1991) Gastrointestinal symptoms in Parkinson’s disease. Mov Disord 6(2):151–156
Jost WH, Eckardt VF (2003) Constipation in idiopathic Parkinson’s disease. Scand J Gastroenterol 38(7):681–686
Ford B (1998) Pain in Parkinson’s disease. Clin Neurosci 5(2):63–72
Aarsland D, Marsh L, Schrag A (2009) Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord 24(15):2175–2186
Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ (2003) Mitochondrial pathology and apoptotic muscle degeneration in drosophila parkin mutants. Proc Natl Acad Sci USA 100(7):4078–4083
Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279(18):18614–18622
Henchcliffe C, Beal MF (2008) Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol 4(11):600–609
Gasser T (2009) Molecular pathogenesis of Parkinson disease: insights from genetic studies. Expert Rev Mol Med 11:e22
Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B (2006) Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of drosophila pink1 is rescued by parkin. Proc Natl Acad Sci USA 103(28):10793–10798
Gautier CA, Kitada T, Shen J (2008) Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci USA 105(32):11364–11369
Sha D, Chin LS, Li L (2010) Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-KappaB signaling. Hum Mol Genet 19(2):352–363
Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW (2008) Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol 7(7):583–590
Smith WW, Pei Z, Jiang H, Moore DJ, Liang Y, West AB, Dawson VL, Dawson TM, Ross CA (2005) Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci USA 102(51):18676–18681
Ng CH, Mok SZ, Koh C, Ouyang X, Fivaz ML, Tan EK, Dawson VL, Dawson TM, Yu F, Lim KL (2009) Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci 29(36):11257–11262
Wang G, van der Walt JM, Mayhew G, Li YJ, Zuchner S, Scott WK, Martin ER, Vance JM (2008) Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet 82(2):283–289
Eacker SM, Dawson TM, Dawson VL (2009) Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10(12):837–841
Acknowledgments
This work was undertaken at UCL/UCLH and was funded in part by the Department of Health NIHR Biomedical Research Centres funding scheme. The Unit of Functional Neurosurgery, UCL Institute of Neurology, Queen Square, London is supported by the Parkinson’s Appeal.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Berry, A.L., Foltynie, T. Gene therapy: a viable therapeutic strategy for Parkinson’s disease?. J Neurol 258, 179–188 (2011). https://doi.org/10.1007/s00415-010-5796-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-010-5796-9