Abstract
Mortality in patients admitted with sepsis is high and the increasing incidence of infections with multiresistant bacteria is a worldwide problem. Many hospitals have local antimicrobial guidelines to assure effective treatment and limit the use of broad-spectrum antibiotics, thereby reducing the selection of resistant bacteria. We evaluated adherence to the antimicrobial treatment guidelines of our hospital in patients presenting to the emergency department (ED) with sepsis and assessed the in vitro susceptibility of isolated pathogens to the guideline-recommended treatment and the prescribed treatment. We included all adult patients with a known or suspected infection and two or more extended systemic inflammatory response syndrome (SIRS) criteria. Patients who did not receive antimicrobial treatment, presented with infections not included in the guidelines, or had more than one possible focus of infection were excluded. A total of 276 ED visits (262 patients) were included. Guideline-concordant treatment was prescribed in 168 visits (61%). In the case of guideline-disconcordant treatment, 87% was more broad-spectrum than guideline-recommended treatment. A microbiological diagnosis was established in 96 visits (35%). The susceptibility of the pathogens isolated from patients treated with guideline-concordant treatment (n = 68) and guideline-disconcordant treatment (n = 28) to guideline-recommended treatment (91% versus 89%) and to prescribed treatment (91% versus 93%) was similar (p = 0.77 and p = 0.79, respectively). In conclusion, non-adherence to the guidelines occurred frequently and resulted in more broad-spectrum empirical therapy. This did not result in a higher rate of susceptibility of the isolated pathogens to the prescribed empirical therapy.
Similar content being viewed by others
Introduction
The mortality rate in patients admitted to the emergency department (ED) with severe sepsis and septic shock is high [1, 2]. Early initiation of appropriate empirical antimicrobial therapy has been shown to improve survival in patients with sepsis and septic shock [3–7]. The choice of the empirical antimicrobial therapy in sepsis mainly depends on the suspected site of infection and the antimicrobial susceptibility of the expected pathogens. To include more resistant but often less prevalent pathogens, the empirical therapy of a severe infection is usually broad-spectrum [4, 8].
Antimicrobial treatment guidelines have been developed to assure effective treatment, decrease treatment diversity, prevent treatment delay, and reduce the unnecessary use of broad-spectrum antimicrobials, thereby reducing the selective pressure on antimicrobial resistance. Due to geographical differences in pathogens and antimicrobial susceptibility, many countries and hospitals have their own antimicrobial treatment guidelines based on local epidemiological data, existing literature, and expert opinion. Although many hospitals have implemented local antimicrobial treatment guidelines, there is a wide variation in the reported adherence to these guidelines [5, 8–10].
The local antimicrobial treatment guidelines in our hospital have been developed, adjusted, and evaluated over the years. The goal of our present study is to evaluate the adherence to these guidelines in patients admitted with sepsis and the in vitro susceptibility of the isolated pathogens to the treatment recommended in the guidelines. When the prescribed antimicrobial therapy deviated from the therapy advised in the guidelines, we compared the susceptibility of the isolated pathogens to the prescribed treatment and the treatment recommended in the guidelines.
Methods
Study setting
This is a retrospective cohort study of patients admitted with sepsis to the ED of the Radboud University Nijmegen Medical Centre, a 950-bed university hospital in the Netherlands. Every year, approximately 20,000 patients visit this ED, which is staffed by residents from the departments of internal medicine (including cardiology, pulmonology, hematology, general internal medicine, geriatrics, oncology, nephrology, gastroenterology, and rheumatology), neurology, and surgery (including orthopedics, urology, and general surgery). Patients admitted to the ED are often referred by their general practitioner to a specific medical speciality, e.g., patients diagnosed with a pneumonia are not exclusively referred to pulmonology but also to other specialities of internal medicine.
All patients (≥16 years old) admitted to the ED between November 6, 2006 and May 9, 2007 with a known or suspected infection and at least two extended systemic inflammatory response syndrome (SIRS) criteria (temperature ≥38.3°C or <36°C, heart rate >90 bpm, respiratory rate >20/min, cold chills, altered mental status, systolic blood pressure <90 mm Hg, mean arterial pressure <65 mm Hg, and hyperglycemia in the absence of diabetes mellitus) were eligible for the study [11]. Patients were excluded if they were diagnosed with an infection not included in the local antimicrobial treatment guidelines, if they did not receive antimicrobial therapy, or if the physician considered >1 specific site of infection (the guidelines do not provide an antimicrobial policy for these situations).
Antimicrobial treatment guidelines
Over the years, the antibiotic committee of our hospital, including a pharmacist, a medical microbiologist, and several clinical specialists, have developed antimicrobial treatment guidelines for the most common types of infection. The first version of these guidelines was introduced more than 10 years ago as a booklet and was distributed among all clinicians throughout the hospital. Since then, many guideline revisions have been made. The latest editions of the guidelines have been available as an easily accessible and easy-to-use electronic version on the hospital intranet, available on every computer in the hospital, and a PDA version can be downloaded. Table 1 is a summary of the guideline recommendations during the study period.
Data collection
Patient demographics, clinical diagnosis with respect to the site of infection, the prescribed antimicrobial therapy at the ED and the medical speciality of the prescribing physician, intensive care unit (ICU) admission within the first 24 h, and length of stay were retrieved from the patient files. The all-cause 30-day mortality was assessed by chart review. When this follow-up was incomplete, the municipal administration and, when necessary, the general practitioner was consulted.
When the physical examination, laboratory results, and imaging results failed to identify a site of infection, the diagnosis was defined as sepsis of unknown origin. As the choice of therapy is based on the clinical diagnosis as well as factors such as where the infection is contracted (community, hospital, nursing home), previous adverse reactions on antimicrobial therapy, prior antimicrobial use, and culture results, the complete medical charts were reviewed for motivations for therapy adjustments in case of guideline-disconcordant treatment [12].
Information about culture collection and culture results was retrieved from the laboratory information system. The clinical significance of culture results was assessed taking into account the clinical information and the quality of the specimen. Bacteria isolated from blood or other sterile body sites were always considered to be significant, except when the isolate is known as a common skin contaminant. In addition, the clinical significance was evaluated by a microbiologist based on the culture results, clinical diagnosis, and response to antimicrobial therapy.
Isolates from sputum were considered to be significant if the sputum sample had <10 squamous cells and >25 leukocytes per low-power field. In patients with a clinical diagnosis of urosepsis, bacteria in concentrations of >105/ml urine were considered to be significant in the presence of leukocyturia without significant epithelial cells. Clinical significance was evaluated from the patient file if bacterial counts were in the range 104–105/ml and in case of a monobacterial culture with bacteria >105/ml in the presence of leukocyturia and epithelial cells. In patients with a diagnosis of skin/wound infection, isolates of true pathogens such as beta-hemolytic streptococci or Staphylococcus aureus were considered to be significant, and the significance of Gram-negative bacteria was determined from investigation of the patient file.
Guideline adherence
The prescribed antimicrobial therapy was divided into “guideline-concordant treatment” and “guideline-disconcordant treatment”. Guideline-concordant treatment was defined as antimicrobial therapy prescribed empirically in accordance with the clinical diagnosis at the ED and the antimicrobial treatment guideline. Complete medical charts were reviewed for motivations for therapy adjustments in case of guideline-discordant treatment. When physicians deviated from the guideline-recommended treatment with good motivation, such as the presence of a known allergy or previously cultured pathogens, the therapy was considered to be guideline-concordant.
Antimicrobial susceptibility
Based on the in vitro susceptibility results (using Clinical and Laboratory Standards Institute [CLSI] breakpoints) of the isolated pathogens, we evaluated the appropriateness of the guideline-recommended treatment as well as the prescribed therapy. Pathogens were considered resistant to antimicrobial therapy when at least one of the isolated micro-organisms categorized as a relevant pathogen was tested resistant by routine in vitro susceptibility testing or was intrinsically resistant to the antimicrobial therapy.
Statistical analysis
We compared the patient demographics and characteristics in patients treated with guideline-concordant treatment and guideline-disconcordant treatment. Categorical variables were analyzed using Pearson’s Chi-squared test and continuous variables were analyzed using Student’s t-test or the Mann–Whitney U-test, as applicable. A p-value of less than 0.05 was considered to be statistically significant. All calculations were performed using SPSS software, version 16.0 for Windows (SPSS Inc., Chicago, IL).
Results
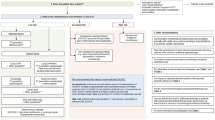
Of a total of 400 ED visits with a known or suspected infection and ≥2 extended SIRS criteria, 276 visits (262 patients) were included in the study (Fig. 1). The mean (± standard deviation [SD]) age was 59 ± 19 years and 63% were male. Blood cultures were positive in 49 patients (18%; contaminated blood cultures not included) and 22 patients were admitted to the ICU within 24 h after admission (8%). The length of stay, ICU admission within 24 h, and 30-day all-cause mortality were similar in patients receiving guideline-concordant and guideline-disconcordant treatment (Table 2). One patient was lost to follow-up.
Antimicrobial treatment guideline adherence
The overall adherence to the guideline-recommended treatment was 61% (Table 3; n = 168). This includes 25 ED visits where the prescribed treatment was considered to be guideline-concordant due to a well-motivated deviation from the guideline-recommended therapy. Adherence was the highest in patients diagnosed with urosepsis and febrile neutropenia (95% and 94%, respectively) and the lowest in patients with pneumonia (43%). Among the patients with pneumonia, adherence was above 50% in patients with severe pneumonia, as defined by the CURB-65 score, patients residing in a nursing home, and patients with recent antibiotic use, whereas adherence in patients with a mild pneumonia was only 34% [13].
In 94 of the 108 patients (87%) with guideline-disconcordant treatment, the antimicrobial therapy was more broad-spectrum than the guideline-recommended therapy, and 66 patients were treated with a beta-lactam/beta-lactamase inhibitor instead of a narrow-spectrum beta-lactam. Treatment diversity was the highest among patients diagnosed with a pneumonia: a total of 12 different antibiotic regimens were prescribed in these patients.
Antimicrobial susceptibility
Positive cultures were found in 133 patients. Thirty-seven cultures were interpreted as non-significant or contamination. These cultures consisted of Candida species, Aspergillus species, or Gram-negative bacteria interpreted as colonization or contamination from 15 urine and 18 sputum specimens, one wound swab with coagulase-negative staphylococci, two blood cultures with coagulase-negative staphylococci, and one Propionibacterium acnes in a biopsy of an intracerebral lesion, later confirmed to be a malignancy. Four cultures that led to a different definite diagnosis than the clinical diagnosis made at the ED were left out of further analysis: two urine cultures diagnostic for urinary tract infection and one Enterococcus faecalis bacteremia of unknown source from patients suspected of pneumonia, and a sputum culture from a patient suspected of meningitis. A final microbiological diagnosis was established in 96 patients (35%). Table 4 shows the pathogens and their susceptibility to guideline-recommended treatment according to the clinical diagnosis.
Of the 96 patients with a microbiological diagnosis, 68 received guideline-concordant treatment. The susceptibility of the isolated pathogens to the guideline-recommended treatment was similar in patients with guideline-concordant treatment and guideline-disconcordant treatment (62/68; 91% and 25/28; 89% respectively, p = 0.77). Furthermore, the susceptibility of the isolated pathogens to the prescribed therapy was similar in patients with guideline-concordant treatment (62/68; 91%) and guideline-disconcordant treatment (26/28; 93%; p = 0.79).
Nine of the 96 isolated pathogens (9%) were resistant to guideline-concordant treatment (Table 4). The percentage of pathogens resistant to guideline-recommended treatment was higher when an ED visit was preceded by a hospitalization in the last 3 months (6/26; 23% versus 3/70; 4%; p = 0.005).
Discussion
During the study period, the overall adherence to our local antimicrobial treatment guidelines in patients admitted to the ED with sepsis was 61%. However, differences between subgroups were substantial, with high adherence rates in patients with urosepsis and febrile neutropenia, and low rates in patients with pneumonia, skin or soft tissue infection, or cholangitis. The empirical therapy in patients treated with guideline-disconcordant treatment was more broad-spectrum than guideline-recommended treatment in the vast majority of patients. However, this use of more broad-spectrum antimicrobial treatment did not result in a higher rate of in vitro susceptibility of the isolated pathogens to the prescribed treatment in the patients with guideline-disconcordant treatment compared to the patients with guideline-concordant treatment. In addition, the isolated pathogens were equally susceptible to the guideline-recommended therapy in both treatment groups. These results indicate that non-compliance to the guideline does not result in a clinical benefit for patients admitted with sepsis.
A small but significant proportion (9%) of isolated pathogens were resistant to the guideline-recommended therapy. These pathogens were mostly cultured from recently hospitalized patients. This is in keeping with an earlier study that identified frequent contacts with the healthcare system, especially recent hospitalization, prior to admission as an important risk factor for ineffective empirical therapy in patients admitted with a bloodstream infection [14]. A more broad-spectrum empirical therapy for this specific group of patients needs to be considered.
Although many hospitals have their own antimicrobial treatment guidelines, little is known about the adherence to these guidelines in patients with sepsis. A high adherence of 90% to local antimicrobial therapy guidelines in patients with a suspected or documented infection (pneumonia, cellulitis or erysipelas, urosepsis, febrile neutropenia, or meningitis) has been described [8]. However, the investigated guidelines were developed by internal medicine specialists for their own use in patients admitted to the internal medicine wards or the ICU. In contrast, we investigated the adherence to local antimicrobial treatment guidelines developed for use in the entire hospital, in patients admitted to the ED and treated by many different physicians and disciplines. Our adherence data are in agreement with other studies that investigated compliance to treatment guidelines in patients admitted with a pneumonia and reported adherence rates of between 41% and 77% [3, 5, 9, 10].
The obvious downside of the unnecessary use of broad-spectrum therapy is the increase in the selective pressure on bacteria, thereby, promoting the emergence of resistant pathogens [15, 16]. Over the last few decades, a dramatic increase of bacterial resistance has emerged with the increasing use of broad-spectrum antimicrobials, whereas, on the other hand, the development of new antimicrobial agents is declining [17]. The use of antimicrobial treatment guidelines based on local epidemiology, followed by de-escalation of the empirical antimicrobial therapy based on culture and susceptibility results, is one of the most important strategies to reduce the use of broad-spectrum antimicrobial therapy and prevent and control the emergence of bacterial resistance. The adherence rate to our local antimicrobial treatment guidelines illustrates the need for ongoing communication about culture and susceptibility results in relation to the prescribed antimicrobial treatment and the antimicrobial treatment guidelines. Previous research has demonstrated that antimicrobial treatment guideline adherence can be improved by close collaboration with representatives of the involved departments and feedback on antimicrobial use in combination with educational training sessions for physicians [18].
Our study has several limitations. First, it was a retrospective cohort study, which implicates that reasons for prescribing guideline-disconcordant treatment were only taken into account when they were recorded in the patients’ medical charts. Furthermore, the study results reflect the epidemiology and guideline adherence of a single center; several subgroups such as patients with cholangitis and meningitis were very small, and the miscellaneous infections were very diverse. However, the goal of our study was to provide an overview of the antimicrobial treatment guideline adherence and the appropriateness of prescribed treatment among all patients admitted to the ED of our hospital with sepsis, and we do believe that our data provide insights into daily clinical practice. The reasons for non-adherence to antimicrobial treatment guidelines were beyond the scope of the current study, but factors identified in other studies will most likely be applicable in our setting [19]. For example, fear for an unfavorable outcome with narrow-spectrum guideline-recommended treatment and a lack of agreement with guidelines have been identified as the main barriers to prescribing empirical antibiotic treatment according to the recommended guidelines in patients with community-acquired pneumonia [20].
Non-adherence to guideline-recommended treatment predominantly resulted in more broad-spectrum empirical therapy. However, pathogens isolated in patients treated with guideline-disconcordant treatment were equally susceptible to guideline-recommended therapy and the actually prescribed treatment. To minimize treatment diversity and the inappropriate use of broad-spectrum antimicrobials, prescribers should be aware that a more broad-spectrum empirical treatment does not result in more effective treatment, but does increase the selection of antimicrobial resistance. A multidisciplinary effort should be made to improve compliance with local antimicrobial treatment guidelines.
Reference
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34(6):1589–1596
Shapiro N, Howell MD, Bates DW, Angus DC, Ngo L, Talmor D (2006) The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Ann Emerg Med 48(5):583–590, 590.e1
Bodí M, Rodríguez A, Solé-Violán J, Gilavert MC, Garnacho J, Blanquer J, Jimenez J, de la Torre MV, Sirvent JM, Almirall J, Doblas A, Badía JR, García F, Mendia A, Jordá R, Bobillo F, Vallés J, Broch MJ, Carrasco N, Herranz MA, Rello J; Community-Acquired Pneumonia Intensive Care Units (CAPUCI) Study Investigators (2005) Antibiotic prescription for community-acquired pneumonia in the intensive care unit: impact of adherence to Infectious Diseases Society of America guidelines on survival. Clin Infect Dis 41(12):1709–1716
Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, Ramsay G, Zimmerman JL, Vincent J-L, Levy MM; Committee Surviving Sepsis Campaign Management Guidelines (2004) Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 32(3):858–873
Frei CR, Attridge RT, Mortensen EM, Restrepo MI, Yu Y, Oramasionwu CU, Ruiz JL, Burgess DS (2010) Guideline-concordant antibiotic use and survival among patients with community-acquired pneumonia admitted to the intensive care unit. Clin Ther 32(2):293–299
Gaieski DF, Mikkelsen ME, Band RA, Pines JM, Massone R, Furia FF, Shofer FS, Goyal M (2010) Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med 38(4):1045–1053
Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD (1998) The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 244(5):379–386
Galayduyk N, Colodner R, Chazan B, Flatau E, Lavi I, Raz R (2008) Adherence to guidelines on empiric use of antibiotics in the emergency room. Infection 36(5):408–414
McCabe C, Kirchner C, Zhang H, Daley J, Fisman DN (2009) Guideline-concordant therapy and reduced mortality and length of stay in adults with community-acquired pneumonia: playing by the rules. Arch Intern Med 169(16):1525–1531
Mortensen EM, Restrepo M, Anzueto A, Pugh J (2004) Effects of guideline-concordant antimicrobial therapy on mortality among patients with community-acquired pneumonia. Am J Med 117(10):726–731
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31(4):1250–1256
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, Lewis SA, Macfarlane JT (2003) Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58(5):377–382
McDonald JR, Friedman ND, Stout JE, Sexton DJ, Kaye KS (2005) Risk factors for ineffective therapy in patients with bloodstream infection. Arch Intern Med 165(3):308–313
Safdar N, Maki DG (2002) The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 136(11):834–844
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL (2000) Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162(2 Pt 1):505–511
Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JE Jr (2004) Trends in antimicrobial drug development: implications for the future. Clin Infect Dis 38(9):1279–1286
Mol PG, Wieringa JE, Nannanpanday PV, Gans RO, Degener JE, Laseur M, Haaijer-Ruskamp FM (2005) Improving compliance with hospital antibiotic guidelines: a time-series intervention analysis. J Antimicrob Chemother 55(4):550–557
Mol PG, Rutten WJ, Gans RO, Degener JE, Haaijer-Ruskamp FM (2004) Adherence barriers to antimicrobial treatment guidelines in teaching hospital, the Netherlands. Emerg Infect Dis 10(3):522–525
Schouten JA, Hulscher ME, Natsch S, Kullberg BJ, van der Meer JW, Grol RP (2007) Barriers to optimal antibiotic use for community-acquired pneumonia at hospitals: a qualitative study. Qual Saf Health Care 16(2):143–149
Acknowledgments
Funding
None.
Competing interests
None declared.
Ethical approval
Not required.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van der Velden, L.B.J., Tromp, M., Bleeker-Rovers, C.P. et al. Non-adherence to antimicrobial treatment guidelines results in more broad-spectrum but not more appropriate therapy. Eur J Clin Microbiol Infect Dis 31, 1561–1568 (2012). https://doi.org/10.1007/s10096-011-1478-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1478-5