Abstract
Purpose
We assessed whether (1) dapagliflozin (Dapa, an SGLT2-inhibitor) attenuates the deterioration of heart function Nlrp3 and inflammasome activation in diabetic mice. (2) The effects can be augmented with saxagliptin (Saxa), a DDP4-inhibitor. (3) Dapa effect is possibly SGLT2-independent on cardiofibroblasts in vitro.
Methods
Type 2 diabetic (BTBR ob/ob) and wild-type (WT) mice received vehicle, Dapa, or Dapa+Saxa for 8 weeks. Glucose tolerance test and echocardiogram were performed. Cardiofibroblasts from WT and BTBR hearts were incubated with Dapa and exposed to LPS.
Results
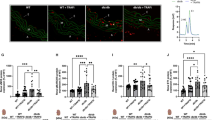
Left ventricular ejection fraction (LVEF) was 81 ± 1% in the WT and 53 ± 1% in the T2D-cont mice. Dapa and Dapa+Saxa improved LVEF to 68 ± 1 and 74.6 ± 1% in the BTBR mice (p < 0.001). The mRNA levels of NALP3, ASC, IL-1β, IL-6, caspase-1, and TNFα were significantly higher in the BTBR compared to the WT hearts; and Dapa and Dapa+Saxa significantly attenuated these levels. Likewise, protein levels of NLRP3, TNFα, and caspase-1 were higher in the BTBR compared to the WT hearts and Dapa, and to a greater extent Dapa+Saxa, attenuated the increase in the BTBR mice. Collagen-1 and collagen-3 mRNA levels significantly increased in the BTBR mice and these increases were attenuated by Dapa and Dapa+Saxa. P-AMPK/total-AMPK ratio was significantly lower in the BTBR mice than in the WT mice. Dapa and Dapa+Saxa equally increased the ratio in the BTBR mice. This in vitro study showed that NALP3, ASC, IL-1β, and caspase-1 mRNA levels were higher in the BTBR cardiofibroblasts and attenuated with Dapa. The effect was AMPK-dependent and SGLT1-independent.
Conclusions
Dapa attenuated the activation of the inflammasome, fibrosis, and deterioration of LVEF in BTBR mice. The anti-inflammatory, anti-fibrotic effects are likely SGLT2- and glucose-lowering-independent, as they were replicated in the in vitro model. The effects on remodeling were augmented when Saxa was added to Dapa. Yet, adding Saxa to Dapa did not result in a greater effect on myocardial fibrosis and collagen levels.








Similar content being viewed by others
References
Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81.
Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22.
Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2015;3:356–66.
Nilsson J, Bengtsson E, Fredrikson GN, Bjorkbacka H. Inflammation and immunity in diabetic vascular complications. Curr Opin Lipidol. 2008;19:519–24.
De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–9.
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904.
Dixit VD. Nlrp3 inflammasome activation in type 2 diabetes: is it clinically relevant? Diabetes. 2013;62:22–4.
Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204.
Anders HJ, Lech M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013;84:225–8.
Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–18.
Luo B, Li B, Wang W, Liu X, Xia Y, Zhang C, et al. NLRP3 gene silencing ameliorates diabetic cardiomyopathy in a type 2 diabetes rat model. PLoS One. 2014;9:e104771.
Luo B, Li B, Wang W, Liu X, Liu X, Xia Y, et al. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther. 2014;28:33–43.
Fuentes-Antras J, Ioan AM, Tunon J, Egido J, Lorenzo O. Activation of toll-like receptors and inflammasome complexes in the diabetic cardiomyopathy-associated inflammation. Int J Endocrinol. 2014;2014:847827.
Singh LP. The NLRP3 inflammasome and diabetic cardiomyopathy: editorial to: “Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model” by Beibei Luo et al. Cardiovasc Drugs Ther. 2014;28:5–6.
Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–29.
Plosker GL. Dapagliflozin: a review of its use in patients with type 2 diabetes. Drugs. 2014;74:2191–209.
Saeed MA, Narendran P. Dapagliflozin for the treatment of type 2 diabetes: a review of the literature. Drug Des Devel Ther. 2014;8:2493–505.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015.
De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis. 2014;64:16–24.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246–55.
Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J Pharm Pharmacol. 2014;66:975–87.
Benetti E, Mastrocola R, Vitarelli G, Cutrin JC, Nigro D, Chiazza F, et al. Empagliflozin protects against diet-induced NLRP-3 inflammasome activation and lipid accumulation. J Pharmacol Exp Ther. 2016;359:45–53.
Birnbaum Y, Bajaj M, Qian J, Ye Y. Dipeptidyl peptidase-4 inhibition by saxagliptin prevents inflammation and renal injury by targeting the Nlrp3/ASC inflammasome. BMJ Open Diabetes Res Care. 2016;4:e000227.
Dai Y, Dai D, Wang X, Ding Z, Mehta JL. DPP-4 inhibitors repress NLRP3 inflammasome and interleukin-1beta via GLP-1 receptor in macrophages through protein kinase C pathway. Cardiovasc Drugs Ther. 2014;28:425–32.
Williams DM, Stephens JW. Combination therapy with saxagliptin and dapagliflozin for the treatment of type 2 diabetes. Expert Opin Pharmacother. 2015;16:2373–9.
Zhou L, Cryan EV, D'Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003;90:339–46.
Vrhovac I, Balen Eror D, Klessen D, Burger C, Breljak D, Kraus O, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467:1881–98.
Chang YK, Choi H, Jeong JY, Na KR, Lee KW, Lim BJ, et al. Dapagliflozin, SGLT2 inhibitor, attenuates renal ischemia-reperfusion injury. PLoS One. 2016;11:e0158810.
Ghosh RK, Bandyopadhyay D, Hajra A, Biswas M, Gupta A. Cardiovascular outcomes of sodium-glucose cotransporter 2 inhibitors: a comprehensive review of clinical and preclinical studies. Int J Cardiol. 2016;212:29–36.
Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100.
Scheen AJ. Reappraisal of the diuretic effect of empagliflozin in the EMPA-REG OUTCOME trial: comparison with classic diuretics. Diabetes Metab. 2016;42:224–33.
Kanwal A, Nizami HL, Mallapudi S, Putcha UK, Mohan GK, Banerjee SK. Inhibition of SGLT1 abrogates preconditioning-induced cardioprotection against ischemia-reperfusion injury. Biochem Biophys Res Commun. 2016;472:392–8.
Kashiwagi Y, Nagoshi T, Yoshino T, Tanaka TD, Ito K, Harada T, et al. Expression of SGLT1 in human hearts and impairment of cardiac glucose uptake by phlorizin during ischemia-reperfusion injury in mice. PLoS One. 2015;10:e0130605.
Tirmenstein M, Dorr TE, Janovitz EB, Hagan D, Abell LM, Onorato JM, et al. Nonclinical toxicology assessments support the chronic safety of dapagliflozin, a first-in-class sodium-glucose cotransporter 2 inhibitor. Int J Toxicol. 2013;32:336–50.
Saito T, Okada S, Yamada E, Shimoda Y, Osaki A, Tagaya Y, et al. Effect of dapagliflozin on colon cancer cell [rapid communication]. Endocr J. 2015;62:1133–7.
Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8.
Millar PJ, Pathak V, Moffett RC, Pathak NM, Bjourson AJ, O'Kane MJ, et al. Beneficial metabolic actions of a stable GIP agonist following pre-treatment with a SGLT2 inhibitor in high fat fed diabetic mice. Mol Cell Endocrinol. 2016;420:37–45.
Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet. 2014;53:17–27.
Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos. 2010;38:405–14.
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK et al. beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016.
Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65:2784–94.
Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol. 2015;65:137–47.
Kornelius E, Lin CL, Chang HH, Li HH, Huang WN, Yang YS, et al. DPP-4 inhibitor linagliptin attenuates Abeta-induced cytotoxicity through activation of AMPK in neuronal cells. CNS Neurosci Ther. 2015;21:549–57.
Ohyama T, Sato K, Yamazaki Y, Hashizume H, Horiguchi N, Kakizaki S, et al. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol. 2014;20:16227–35.
Murase H, Kuno A, Miki T, Tanno M, Yano T, Kouzu H, et al. Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc Diabetol. 2015;14:103.
Lenski M, Kazakov A, Marx N, Bohm M, Laufs U. Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol. 2011;51:906–18.
Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 inhibitors and cardiovascular risk: lessons learned from the EMPA-REG OUTCOME study. Diabetes Care. 2016;39:717–25.
Daniele G, Xiong J, Solis-Herrera C, Merovci A, Eldor R, Tripathy D, et al. Dapagliflozin enhances fat oxidation and ketone production in patients with type 2 diabetes. Diabetes Care. 2016;39:2036–41.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by an investigator initiated grant from AstraZeneca and the John S. Dunn Chair in Cardiology Research and Education.
Conflict of Interest
Dr. Ye received research grants from Astra Zeneca and Boehringer Ingelheim. Dr. Bajaj received research grants from AstraZeneca, Boehringer Ingelheim, Eli-Lilly, and Novo Nordisk. He has received lecture fees from Takeda Pharmaceuticals and Sanofi Aventis and is a consultant to Merck and Genentech. Dr. Yang is an employee of Astra Zeneca. Dr. Perez-Polo reports no conflict of interest. Dr. Yochai Birnbaum received research grants from Astra Zeneca and he is a speaker for AstraZeneca.
Research Involving Animals
Mice received humane care in compliance with “The Guide for the Care and Use of Laboratory Animals” published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996). The protocol was approved by the University of Texas Medical Branch IACUC, Galveston, Texas, USA.
Informed Consent
Not applicable.
Rights and permissions
About this article
Cite this article
Ye, Y., Bajaj, M., Yang, HC. et al. SGLT-2 Inhibition with Dapagliflozin Reduces the Activation of the Nlrp3/ASC Inflammasome and Attenuates the Development of Diabetic Cardiomyopathy in Mice with Type 2 Diabetes. Further Augmentation of the Effects with Saxagliptin, a DPP4 Inhibitor. Cardiovasc Drugs Ther 31, 119–132 (2017). https://doi.org/10.1007/s10557-017-6725-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-017-6725-2