Abstract
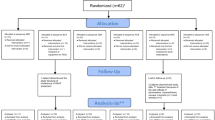
Ramelteon is a selective MT1/MT2-receptor agonist indicated for insomnia treatment. Because it has no depressant effects on the nervous system, it is not expected to affect the control of breathing. The potential effects of ramelteon on apneic and hypopneic events and arterial oxygen saturation (SaO2) in individuals with obstructive sleep apnea were assessed. In this double-blind, randomized, crossover study, 26 adults with mild to moderate obstructive sleep apnea received ramelteon 16 mg and placebo for one night each, with a 5- to 12-day washout period between treatments. Treatments were administered 30 min before habitual bedtime. Respiratory effort was monitored using respiratory inductance plethysmography, SaO2 was measured by pulse oximetry, and sleep onset and duration were measured by polysomnography and post-sleep questionnaire. Post-sleep questionnaire also measured next-day residual effects. The primary measure was apnea–hypopnea index. Apnea–hypopnea index was similar in ramelteon and placebo groups (11.4 vs 11.1, respectively; CI = −2.1, 2.6, P = 0.812). Ramelteon had no effect on the number of central, obstructive, or mixed apnea episodes. No significant differences were observed in SaO2 for the entire night between ramelteon and placebo (95.1 vs 94.7%; P = 0.070). Ramelteon did not meaningfully affect sleep when evaluated by polysomnography and post-sleep questionnaire. Compared with placebo, ramelteon had no significant effect on next-day residual effects. Adverse events were reported by three subjects in the ramelteon group: headache (n = 2) and urinary tract infection (n = 1). No adverse events were reported with placebo. Ramelteon was well-tolerated and, as expected, did not worsen sleep apnea when administered to subjects with mild to moderate obstructive sleep apnea.
Similar content being viewed by others
References
Smith S, Sullivan K, Hopkins W, Douglas J (2004) Frequency of insomnia reports in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 5:449–456
Krakow B, Melendrez D, Ferreira E, Clark J, Warner TD, Sisley B, Sklar D (2001) Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 120:1923–1929
Shepertycky MR, Banno K, Kryger MH (2005) Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep 28;309–314
George CF (2000) Perspectives on the management of insomnia in patients with chronic respiratory disorders. Sleep 23(Suppl 1):S31–S35
Guilleminault C (1990) Benzodiazepines, breathing, and sleep. Am J Med 88:25S–28S
Berry RB, Kouchi K, Bower J, Prosise G, Light RW (1995) Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med 151:450–454
Kato K, Hirai K, Nishiyama K, Uchikawa O, Fukatsu K, Ohkawa S, Kawamata Y, Hinuma S, Miyamoto M (2005) Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48:301–310
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19:91–102
von Gall C, Stehle JH, Weaver DR (2002) Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res 309:151–162
Erman M, Seiden D, Zammit G, Sainati S, Zhang J (2006) An efficacy, safety, and dose–response study of ramelteon in patients with chronic primary insomnia. Sleep Med 7:17–24
Zammit G, Roth T, Erman M, Sainati S, Weigand S, Zhang J (2005) Double-blind, placebo-controlled polysomnography and outpatient trial to evaluate the efficacy and safety of ramelteon in adult patients with chronic insomnia. Sleep 28:A228–A229
Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P (2006) Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med 7:312–318
Roth T, Stubbs C, Walsh J (2005) Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 28:303–307
Johnson M, Suess P, Griffiths RR (2006) Ramelteon: a novel hypnotic lacking abuse liability and sedative side effects. Arch Gen Psychiatry 63:1149–1157
Dubocovich ML, Yun K, Al-Ghoul WM, Benloucif S, Masana MI (1998) Selective MT2 melatonin receptor antagonists block melatonin-mediated phase advances of circadian rhythms. FASEB J 12:1211–1220
Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR (2003) Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 23:1054–1060
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Acknowledgements
This study was funded by Takeda Pharmaceutical Company Limited.
Author Financial Disclosure
Meir Kryger, MD: Consultant, Takeda Pharmaceuticals North America, Inc.
Thomas Roth, PhD: Grants and Consultant, Takeda Pharmaceuticals North America, Inc.
Sherry Wang-Weigand, MD, PhD: Employee, Takeda Global Research & Development Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kryger, M., Wang-Weigand, S. & Roth, T. Safety of ramelteon in individuals with mild to moderate obstructive sleep apnea. Sleep Breath 11, 159–164 (2007). https://doi.org/10.1007/s11325-006-0096-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-006-0096-4