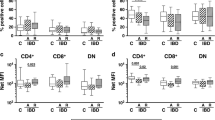
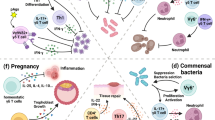
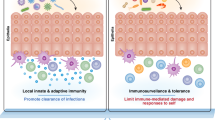
Abstract
Active inflammatory bowel disease (IBD) is often associated with simultaneous inflammation in the skin, eyes and joints. Inflammatory disease in the liver can also occur in patients with IBD but seems to be independent of inflammation in the bowel. In this Opinion article, we propose that the hepatic complications of IBD are mediated by long-lived mucosal T cells that are recruited to the liver in response to aberrantly expressed endothelial-cell adhesion molecules and chemokines that are normally restricted to the gut. Similar mechanisms might explain why certain diseases are associated with site-specific tissue distributions and might point to new therapeutic strategies that are based on modulating tissue-specific lymphocyte homing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Podolsky, D. K. Inflammatory bowel disease (2). N. Engl. J. Med. 325, 1008–1016 (1991).
Vierling, J. M. in Hepatology: a Textbook of liver disease, 4th edn; Ch. 40 (eds Zakim, D. & Boyer, T.D.) 1221–1272 (W.B. Saunders, Philadelphia, 2003).
Orchard, T. R., Wordsworth, B. P. & Jewell, D. P. Peripheral arthropathies in inflammatory bowel disease: their articular distribution and natural history. Gut 42, 387–391 (1998).
Camus, P., Piard, F., Ashcroft, T., Gal, A. A. & Colby, T. V. The lung in inflammatory bowel disease. Medicine (Baltimore) 72, 151–183 (1993).
Broome, U. et al. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut 38, 610–615 (1996).
Chapman, R. W. Aetiology and natural history of primary sclerosing cholangitis — a decade of progress? Gut 32, 1433–1435 (1991).
Cuoco, L. et al. Onset of ulcerative colitis during immunosuppressive therapy for liver transplantation. Am. J. Gastroenterol. 92, 2134–2135 (1997).
Cullen, S. & Chapman, R. Primary sclerosing cholangitis. Autoimmun. Rev. 2, 305–312 (2003).
Salmi, M., Andrew, D. P., Butcher, E. C. & Jalkanen, S. Dual binding capacity of mucosal immunoblasts to mucosal and synovial endothelium in humans: dissection of the molecular mechanisms. J. Exp. Med. 181, 137–149 (1995).
Butcher, E. C. & Picker, L. J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Springer, T. A. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu. Rev. Physiol. 57, 827–872 (1995).
Ley, K. & Kansas, G. S. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nature Rev. Immunol. 4, 325–335 (2004).
Tanaka, Y. et al. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature 361, 79–82 (1993).
Cinamon, G., Shinder, V. & Alon, R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nature Immunol. 2, 515–522 (2001).
Miyasaka, M. & Tanaka, T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nature Rev. Immunol. 4, 360–370 (2004).
Aurrand-Lions, M. et al. Junctional adhesion molecule-C regulates the early influx of leukocytes into tissues during inflammation. J. Immunol. 174, 6406–6415 (2005).
Johnson-Leger, C. & Imhof, B. A. Forging the endothelium during inflammation: pushing at a half-open door? Cell Tissue Res. 314, 93–105 (2003).
Parsonage, G. et al. A stromal address code defined by fibroblasts. Trends Immunol. 26, 150–156 (2005).
Edwards, S., Lalor, P. F., Nash, G. B., Rainger, G. E. & Adams, D. H. Lymphocyte traffic through sinusoidal endothelial cells is regulated by hepatocytes. Hepatology 41, 451–459 (2005).
von Andrian, U. H. & Mackay, C. R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
MacDonald, T. T. & Monteleone, G. Immunity, inflammation, and allergy in the gut. Science 307, 1920–1925 (2005).
Mowat, A. M. Anatomical basis of tolerance and immunity to intestinal antigens. Nature Rev. Immunol. 3, 331–341 (2003).
Johansson-Lindbom, B. et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J. Exp. Med. 202, 1063–1073 (2005).
Gunn, M. D. et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl Acad. Sci. USA 95, 258–263 (1998).
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Berg, E. L., McEvoy, L. M., Berlin, C., Bargatze, R. F. & Butcher, E. C. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature 366, 695–698 (1993).
von Andrian, U. H. & Mempel, T. R. Homing and cellular traffic in lymph nodes. Nature Rev. Immunol. 3, 867–878 (2003).
Briskin, M. et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 151, 97–110 (1997).
Briskin, M. J., McEvoy, L. M. & Butcher, E. C. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature 363, 461–464 (1993).
Shyjan, A. M., Bertagnolli, M., Kenney, C. J. & Briskin, M. J. Human mucosal addressin cell adhesion molecule-1 (MAdCAM-1) demonstrates structural and functional similarities to the α4β7-integrin binding domains of murine MAdCAM-1, but extreme divergence of mucin-like sequences. J. Immunol. 156, 2851–2857 (1996).
Mora, J. R. et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature 424, 88–93 (2003).
Stagg, A. J., Kamm, M. A. & Knight, S. C. Intestinal dendritic cells increase T cell expression of α4β7 integrin. Eur. J. Immunol. 32, 1445–1454 (2002).
Johansson-Lindbom, B. et al. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J. Exp. Med. 198, 963–969 (2003).
Johansen, F. E. et al. Regional induction of adhesion molecules and chemokine receptors explains disparate homing of human B cells to systemic and mucosal effector sites: dispersion from tonsils. Blood 106, 593–600 (2005).
Iwata, M. et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004).
Hesterberg, P. E. et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin α4β7 . Gastroenterology 111, 1373–1380 (1996).
Ghosh, S. et al. Natalizumab for active Crohn's disease. N. Engl. J. Med. 348, 24–32 (2003).
Kunkel, E. J. et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 (2000).
Vicari, A. P. et al. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity 7, 291–301 (1997).
Papadakis, K. A. et al. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165, 5069–5076 (2000).
Hosoe, N. et al. Demonstration of functional role of TECK/CCL25 in T lymphocyte-endothelium interaction in inflamed and uninflamed intestinal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G458–G466 (2004).
Eksteen, B. et al. Hepatic endothelial CCL25 mediates the recruitment of CCR9+ gut-homing lymphocytes to the liver in primary sclerosing cholangitis. J. Exp. Med. 200, 1511–1517 (2004).
Wurbel, M. A. et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood 98, 2626–2632 (2001).
Wagner, N. et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 382, 366–370 (1996).
Papadakis, K. A. et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology 121, 246–254 (2001).
Lundqvist, K. & Broome, U. Differences in colonic disease activity in patients with ulcerative colitis with and without primary sclerosing cholangitis: a case control study. Dis. Colon Rectum 40, 451–456 (1997).
Loftus E.V. Jr et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut 54, 91–96 (2005).
Berg, E. L. et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 174, 1461–1466 (1991).
Campbell, J. J. et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 (1999).
Reiss, Y., Proudfoot, A. E., Power, C. A., Campbell, J. J. & Butcher, E. C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 194, 1541–1547 (2001).
Homey, B. et al. CCL27–CCR10 interactions regulate T cell-mediated skin inflammation. Nature Med. 8, 157–165 (2002).
Schaerli, P. et al. A skin-selective homing mechanism for human immune surveillance T cells. J. Exp. Med. 199, 1265–1275 (2004).
Rott, L. S. et al. Expression of mucosal homing receptor α4β7 by circulating CD4+ cells with memory for intestinal rotavirus. J. Clin. Invest. 100, 1204–1208 (1997).
Williams, M. B. et al. The memory B cell subset responsible for the secretory IgA response and protective humoral immunity to rotavirus expresses the intestinal homing receptor, α4β7 . J. Immunol. 161, 4227–4235 (1998).
Mora, J. R. et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 201, 303–316 (2005).
Dudda, J. C. et al. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur. J. Immunol. 35, 1056–1065 (2005).
Salmi, M., Kalimo, K. & Jalkanen, S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J. Exp. Med. 178, 2255–2260 (1993).
Agace, W. W. et al. Human intestinal lamina propria and intraepithelial lymphocytes express receptors specific for chemokines induced by inflammation. Eur. J. Immunol. 30, 819–826 (2000).
Soriano, A. et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab. Invest. 80, 1541–1551 (2000).
Strober, W., Fuss, I. J. & Blumberg, R. S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 20, 495–549 (2002).
Crispe, I. N. Hepatic T cells and liver tolerance. Nature Rev. Immunol. 3, 51–62 (2003).
Kenna, T. et al. NKT cells from normal and tumor-bearing human livers are phenotypically and functionally distinct from murine NKT cells. J. Immunol. 171, 1775–1779 (2003).
Doherty, D. G. et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J. Immunol. 163, 2314–2321 (1999).
Ward, S. M. et al. Virus-specific CD8+ T lymphocytes within the normal human liver. Eur. J. Immunol. 34, 1526–1531 (2004).
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001).
Marzo, A. L., Vezys, V., Williams, K., Tough, D. F. & Lefrancois, L. Tissue-level regulation of Th1 and Th2 primary and memory CD4 T cells in response to Listeria infection. J. Immunol. 168, 4504–4510 (2002).
Gowans, J. L. & Knight, E. J. The route of recirculation of lymphocytes in the rat. Proc. R. Soc. Lond. B. 159, 257–282 (1964).
Klonowski, K. D. et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562 (2004).
Murai, M. et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nature Immunol. 4, 154–160 (2003).
Petrovic, A. et al. LPAM (α4β7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood 103, 1542–1547 (2004).
Grant, A. J., Lalor, P. F., Hubscher, S. G., Briskin, M. & Adams, D. H. MAdCAM-1 expressed in chronic inflammatory liver disease supports mucosal lymphocyte adhesion to hepatic endothelium (MAdCAM-1 in chronic inflammatory liver disease). Hepatology 33, 1065–1072 (2001).
Goddard, S., Youster, J., Morgan, E. & Adams, D. H. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am. J. Pathol. 164, 511–519 (2004).
Lian, Z. X. et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J. Immunol. 170, 2323–2330 (2003).
Bowen, D. G. et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J. Clin. Invest. 114, 701–712 (2004).
Knolle, P. A. & Limmer, A. Neighborhood politics: the immunoregulatory function of organ-resident liver endothelial cells. Trends Immunol. 22, 432–437 (2001).
Kudo, S., Matsuno, K., Ezaki, T. & Ogawa, M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J. Exp. Med. 185, 777–784 (1997).
Uwatoku, R. et al. Kupffer cell-mediated recruitment of rat dendritic cells to the liver: roles of N-acetylgalactosamine-specific sugar receptors. Gastroenterology 121, 1460–1472 (2001).
Yoneyama, H. et al. Regulation by chemokines of circulating dendritic cell precursors, and the formation of portal tract-associated lymphoid tissue, in a granulomatous liver disease. J. Exp. Med. 193, 35–49 (2001).
Abe, M., Zahorchak, A. F., Colvin, B. L. & Thomson, A. W. Migratory responses of murine hepatic myeloid, lymphoid-related, and plasmacytoid dendritic cells to CC chemokines. Transplantation 78, 762–765 (2004).
Lalor, P. F., Shields, P., Grant, A. & Adams, D. H. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80, 52–64 (2002).
Smedsrod, B. et al. Cell biology of liver endothelial and Kupffer cells. Gut 35, 1509–1516 (1994).
Matsumoto, T. & Kawakami, M. The unit-concept of hepatic parenchyma—a re-examination based on angioarchitectural studies. Acta Pathol. Jpn 32 (Suppl. 2), 285–314 (1982).
Desmet, V. J., Gerber, M., Hoofnagle, J. H., Manns, M. & Scheuer, P. J. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 19, 1513–1520 (1994).
Wong, J. et al. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Invest. 99, 2782–2790 (1997).
Steinhoff, G., Behrend, M., Schrader, B., Duijvestijn, A. M. & Wonigeit, K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia- lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2 and LFA-3. Am. J. Pathol. 142, 481–488 (1993).
Scoazec, J. Y. & Feldmann, G. The cell adhesion molecules of hepatic sinusoidal endothelial cells. J. Hepatol. 20, 296–300 (1994).
McNab, G. et al. Vascular adhesion protein 1 mediates binding of T cells to human hepatic endothelium. Gastroenterology 110, 522–528 (1996).
Lalor, P. F. et al. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J. Immunol. 169, 983–992 (2002).
Bonder, C. S. et al. Rules of recruitment for Th1 and Th2 lymphocytes in inflamed liver: a role for α4 integrin and vascular adhesion protein-1. Immunity 23, 153–163 (2005).
Boisvert, J. et al. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J. Hepatol. 38, 67–75 (2003).
Shields, P. L. et al. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J. Immunol. 163, 6236–6243 (1999).
Murai, M. et al. Active participation of CCR5+CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Invest. 104, 49–57 (1999).
Coulomb-L'Hermin, A. et al. Stromal cell-derived factor 1 (SDF-1) and antenatal human B cell lymphopoiesis: expression of SDF-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc. Natl Acad. Sci. USA 96, 8585–8590 (1999).
Heydtmann, M. et al. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 174, 1055–1062 (2005).
Isse, K. et al. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology 41, 506–516 (2005).
Narumi, S. et al. Expression of IFN-inducible protein-10 in chronic hepatitis. J. Immunol. 158, 5536–5544 (1997).
Sato, T. et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J. Immunol. 174, 277–283 (2005).
Geissmann, F. et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 3, e113 (2005).
Curbishley, S. M., Eksteen, B., Gladue, R. P., Lalor, P. & Adams, D. H. CXCR3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am. J. Pathol. 167, 887–899 (2005).
Arvilommi, A. M., Salmi, M., Kalimo, K. & Jalkanen, S. Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1). Eur. J. Immunol. 26, 825–833 (1996).
Salmi, M., Rajala, P. & Jalkanen, S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J. Clin. Invest. 99, 2165–2172 (1997).
Grant, A. J., Lalor, P. F., Salmi, M., Jalkanen, S. & Adams, D. H. Homing of mucosal lymphocytes to the liver in the pathogenesis of hepatic complications of inflammatory bowel disease. Lancet 359, 150–157 (2002).
Connor, E. M., Eppihimer, M. J., Morise, Z., Granger, D. N. & Grisham, M. B. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J. Leukoc. Biol. 65, 349–355 (1999).
Hillan, K. J. et al. Expression of the mucosal vascular addressin, MAdCAM-1, in inflammatory liver disease. Liver 19, 509–518 (1999).
Salter-Cid, L. M. et al. Anti-inflammatory effects of inhibiting the amine oxidase activity of semicarbazide-sensitive amine oxidase. J. Pharmacol. Exp. Ther. 315, 553–562 (2005).
Zabel, B. A. et al. Human G protein-coupled receptor GPR-9–6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190, 1241–1256 (1999).
Grant, A. J. et al. Hepatic expression of secondary lymphoid chemokine (CCL21) promotes the development of portal-associated lymphoid tissue in chronic inflammatory liver disease. Am. J. Pathol. 160, 1445–1455 (2002).
Kratz, A., Campos-Neto, A., Hanson, M. S. & Ruddle, N. H. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 183, 1461–1472 (1996).
Alexander, J. S. & Ando, T. Density-dependent control of MAdCAM-1 and chronic inflammation. Focus on “Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells”. Am. J. Physiol. Cell Physiol. 288, C243–C244 (2005).
Fu, M. et al. Egr-1 target genes in human endothelial cells identified by microarray analysis. Gene 315, 33–41 (2003).
Acknowledgements
We would like to thank A. Grant, A. Miles, S. Curbishley and P. Lalor, who have contributed to work discussed in this article, and M. Briskin, S. Jalkanen and M. Salmi, who have contributed intellectually to the development of the ideas. B.E. is funded by Fellowships from Core and the Medical Research Council, UK, and the work has also been supported by funding from the European Commission, the Wellcome Trust and a research grant from Pfizer Inc. We would also like to thank C. Buckley, A. Rickinson and M. Salmon for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Autoimmune hepatitis
-
Persistent inflammation of the liver that is characterized by interface hepatitis, hypergammaglobulinaemia and autoantibodies in the serum.
- Endothelins
-
A family of peptides (endothelin 1, endothelin 2 and endothelin 3) that are produced in various tissues. They function as modulators of vasomotor tone, cell proliferation and hormone production.
- Porta hepatis
-
A transverse fissure on the abdominal surface of the liver, where the portal vein, hepatic artery and hepatic ducts enter the liver. Also known as the hilum.
- Primary sclerosing cholangitis
-
A persistent chronic inflammatory disease that is focused in the intrahepatic and extrahepatic bile ducts and is often associated with inflammatory bowel disease.
- Sieve plates
-
A cluster of small holes (fenestrae) in a liver sinusoidal endothelial cell, which is thought to facilitate the diffusion of molecules between the hepatic sinusoid and the underlying space of Disse, which is where solutes can interact with hepatocytes.
- Space of Disse
-
Located between the hepatocytes and the sinusoid, the space of Disse contains hepatic stellate cells (myofibroblasts) and a network of reticular fibres that hold the hepatocytes together. Microvilli that extend from the hepatocytes increase the surface area exposed in the space of Disse.
- Thromboxanes
-
Arachidonic-acid metabolites that are produced by the action of thromboxane synthetase on prostaglandin cyclic endoperoxides. They cause platelet aggregation, vasoconstriction and have pro-inflammatory properties.
- Weibel–Palade bodies
-
(WPBs). Rod-shaped secretory granules that are found in endothelial cells. Exocytosis of WPBs in response to pro-inflammatory stimuli delivers the adhesive protein von Willebrand factor and the leukocyte adhesion molecule platelet selectin to the endothelial-cell surface, where they have important roles in vascular haemostasis and inflammation.
Rights and permissions
About this article
Cite this article
Adams, D., Eksteen, B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 6, 244–251 (2006). https://doi.org/10.1038/nri1784
Issue Date:
DOI: https://doi.org/10.1038/nri1784
This article is cited by
-
Gut immune cell trafficking: inter-organ communication and immune-mediated inflammation
Nature Reviews Gastroenterology & Hepatology (2023)
-
Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs
Nature Reviews Materials (2023)
-
Challenges and opportunities in achieving effective regulatory T cell therapy in autoimmune liver disease
Seminars in Immunopathology (2022)
-
MAdCAM-1 mediates retinal neuron degeneration in experimental colitis through recruiting gut-homing CD4+ T cells
Mucosal Immunology (2021)
-
Vedolizumab and Extraintestinal Manifestations in Inflammatory Bowel Disease
Drugs (2021)