-
PDF
- Split View
-
Views
-
Cite
Cite
Duane J. Gubler, The Continuing Spread of West Nile Virus in the Western Hemisphere, Clinical Infectious Diseases, Volume 45, Issue 8, 15 October 2007, Pages 1039–1046, https://doi.org/10.1086/521911
Close - Share Icon Share
Abstract
West Nile virus (WNV) has historically been considered to be among the least virulent of the Japanese serogroup viruses of the family Flaviviridae, genus Flavivirus. However, recent epidemics associated with severe and fatal neuroinvasive disease have changed that perception. The emergence of a virus subtype with greater epidemic potential and virulence in the early 1990s facilitated the geographic expansion and westward spread of WNV; in 1999, it first appeared in the western hemisphere. Because of the broad host and vector range, the virus has become established in much of the region, and there is little chance that it will be eliminated. Transmission is difficult to predict and even more difficult to prevent and control. The cost-effectiveness of human WNV vaccines is uncertain. The building of laboratory diagnostic, epidemiologic, and vector-control capacity in WNV-enzootic countries is critical to the development of effective prevention and control strategies for WNV infection, as well as for other potential emerging vectorborne viral diseases.
West Nile virus (WNV) belongs to the family Flaviviridae, genus Flavivirus [1]. It is a member of the Japanese encephalitis virus serogroup of flaviviruses, which includes a number of closely related viruses that also cause human disease, including Japanese encephalitis in Asia, St. Louis encephalitis in the Americas, and Murray Valley encephalitis in Australia. All have a similar transmission cycle, with birds serving as the natural vertebrate host and Culex species mosquitoes serving as the enzootic and/or epizootic vectors. All of the aforementioned viruses can infect humans and domestic animals, such as horses, which are generally thought to be incidental hosts.
The presentation of clinical illness in humans varies from asymptomatic infection to viral syndrome to neurologic disease [2]. In recent epidemics of WNV infection, ∼80% of infections were asymptomatic; ∼20% presented as a dengue-like viral syndrome, which can be severe but which is generally self-limited; and <1% led to neuroinvasive disease. The latter cases include encephalitis, meningitis, and polio-like flaccid paralysis [2]. The incubation period may be as short as 2 days or as long as 14 days. A total of 25%–50% of patients will have a rash. Case-fatality rates have ranged from 4% to 15%; patients with encephalitis and flaccid paralysis who survive, however, have a poor prognosis, and many experience long-lasting or permanent neurologic sequelae. WNV has historically been considered as the least virulent of the 4 Japanese serogroup viruses mentioned above, but recent epidemics of disease have changed that perception.
History
WNV was first isolated from a blood specimen obtained from a febrile patient in the West Nile province of Uganda in 1937 [3]. From that time until the fall of 1999, WNV was considered to be relatively unimportant as a human and animal pathogen. The virus was enzootic throughout Africa, west and central Asia, the Middle East, and the Mediterranean, with occasional extension into Europe [4]. A subtype of WNV (Kunjin) is also found in Australia. A characteristic of WNV epidemiology during the period from 1937 to 1999 was that epidemics of infection occurred only occasionally, and infection in humans, horses, and birds was generally either asymptomatic or mild; neurologic disease and death were rare [4–7]. One exception was a small outbreak of apparent WNV infection associated with severe and fatal neurologic disease in both horses and humans in the Camargue region of France in the 1960s [8–10]. The long interval between epidemics, the perception that WNV was not of great public health importance, and lack of reporting of epidemics and/or epizootics in the Mediterranean region all helped mask the emergence of epidemic WNV infection associated with severe and fatal neurologic disease in the 1990s.
Wnv in the Western Hemisphere
In late August 1999, an astute physician in Queens, New York, recognized an unusual cluster of elderly patients with viral encephalitis [11]. Because of the age group involved and the clinical presentation, these cases were initially thought to be St. Louis encephalitis, but subsequent serologic and virologic investigation revealed that the cases were caused by WNV [12]. The epidemic investigation identified 62 cases, with 59 cases of neuroinvasive disease and 7 deaths (12%) [13]. Subsequent epidemiologic studies, however, suggested widespread transmission in New York City, with thousands of infections in the city [14]. Sequence data from the infecting virus suggested that it was imported from the Middle East, most likely from Israel [12]. Several sources of introduction were investigated, including infected humans, birds, and mosquitoes and intentional release. Although it will never be known for sure, the epidemiologic evidence suggests that the virus was introduced in the spring or early summer of 1999, most likely via infected humans arriving from Israel, which was experiencing an epidemic of WNV infection in Tel Aviv at the time [15].
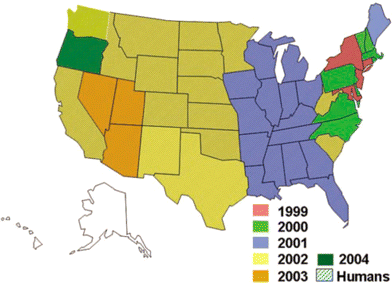
In 1999, WNV spread throughout the states of New York, Connecticut, and New Jersey, and a single infected dead bird was found in Baltimore, Maryland [2]. In 2000, there were only 21 human cases reported (19 cases of neuroinvasive disease and 2 deaths [11%]), all of them in New York, although dead bird surveillance detected WNV in 12 states along the Atlantic coast as far south as North Carolina (figure 1) [16]. However, WNV was detected in Florida in early 2001, suggesting that the virus had spread farther south in 2000 but had not been detected. In 2001, WNV was detected in 21 states, having spread into the east central states as far west as Iowa and Louisiana and into Ontario, Canada. In 2001, there were still relatively few human cases reported (n = 66), including cases of 64 neuroinvasive disease and 9 deaths (14%). In 2002 and 2003, the virus continued to move westward, reaching California and causing the largest epidemics of arboviral meningoencephalitis in the history of the country [2]. In 2002, the epicenters of transmission were in the north central states and in Louisiana—a distribution similar to that of the large St. Louis encephalitis epidemic in 1975 [17]. There were 4156 cases reported in the United States, with 2946 cases of neuroinvasive disease and 284 deaths (10%) [2]. In Canada, the virus occurred in 5 southern provinces along the northern border of the United States, with 426 human cases reported primarily from Ontario, but with cases also reported from Quebec [18]. In 2003, the epicenter of transmission moved farther west to the central plains states. There were 9862 cases reported, with 2866 cases of neuroinvasive disease and 264 fatalities (10%) in the United States. In Canada, the virus was reported in all provinces bordering the Unites States, with the exception of British Columbia; 1495 cases were reported, the majority of which (n = 848) occurred in Saskatchewan. In 2004, transmission waned somewhat, with 2539 cases, including 1142 cases of neuroinvasive disease, and 100 deaths (9%), reported in the United States and only 25 cases reported in Canada [19]. The epicenter of transmission was in California and in the intermountain states. In 2005, 3000 cases were reported; there were 1294 cases of neuroinvasive disease and 119 deaths (9%); in 2006, the number of reported cases increased to 4261, with 1455 cases of neuroinvasive disease and 194 deaths (12%) in the United States. In Canada, 229 and 151 cases were reported in 2005 and 2006, respectively [19] (Harvey Artsob, personal communication).
The sequential westward movement of West Nile virus in the United States, by year, 1999–2004. Data are as of 31 January 2006. Only Oregon and Maine have not experienced human cases. Figure appears courtesy of the Centers for Disease Control and Prevention.
There was also a large epizootic of WNV encephalitis in equines in 2002, with 14,571 cases reported and a case-fatality rate approaching 30%. The number of equine cases decreased dramatically in 2003 and 2004 because of the widespread use of an equine vaccine for WNV. The virus has apparently become enzootic in all parts of the United States, although a lack of reporting may be affecting the accuracy of dead bird surveillance. Serologic surveys conducted in several areas of intense transmission, however, revealed a low prevalence of humans with WNV antibodies present; thus, future epidemics of human disease can be expected.
WNV was first detected south of the US border in 2001, when a human case of neuroinvasive disease was reported in the Cayman Islands [20], and birds collected in Jamaica in early 2002 tested positive for WNV neutralizing antibodies [21]. In 2002, WNV activity was reported in birds and/or equines in Mexico (in 6 states) and on the Caribbean islands of Hispaniola (Greater Antilles) and Guadeloupe (Eastern Antilles). Most likely, the virus was present in Mexico in 2001, because a cow with WNV neutralizing antibody was detected in the southern state of Chiapas in July 2001 [22]. In 2003, the virus was detected in 22 states of Mexico; in Belize, Guatemala, and El Salvador in Central America; and in Cuba, Puerto Rico, and the Bahamas in the Caribbean. In 2004, WNV activity was reported from northern Colombia and from Trinidad—the first reported activity in South America. In 2006, Argentina reported WNV transmission [23].
Of interest is the puzzling fact that severe neurologic and fatal disease associated with WNV infection has been rare in humans and equines in the Caribbean or in Central or South America. This was not unexpected, however, because both Japanese encephalitis virus and St. Louis encephalitis virus exhibit a similar epidemiology: epidemics associated with severe neuroinvasive disease occur mostly in the temperate regions of their geographic distribution, although transmission in general occurs widely in tropical countries of Asia and the Americas. Likewise, in both tropical and temperate regions of the Old World, WNV infection has a similar epidemiology.
The reason for this type of epidemiologic pattern among the very closely related Japanese encephalitis serogroup viruses is complex and not well understood. The viruses all have a similar transmission cycle, with birds and Culex species mosquitoes as their principal vertebrate and vector hosts, respectively, and all are spread by migratory birds. There are several hypotheses that might provide an explanation. First, it is possible that birds infected with the more virulent epidemic strains of virus are too sick to migrate; thus, only birds infected with less virulent viral strains make the long journey south [21]. However, a number of bird species that have high WNV loads do not develop severe or fatal disease and are likely capable of migrating [24]. Moreover, recent experimental infection of migratory birds has shown that WNV infection does not inhibit migratory behavior and that migratory status did not affect virus titers [25]. Finally, WNV-infected storks that migrated from Africa were found in Israel [26].
A second hypothesis to explain why more humans and equines do not develop severe neurologic disease in tropical areas of their distribution involves cross-reactive flavivirus antibody. There are numerous endemic and/or enzootic flaviviruses in the Caribbean and in Central and South America, including Dengue, St. Louis encephalitis, yellow fever, Ilheus, Roccio, Cacipacore, Aroa, Naranjal, Bussuguara, and Iguape viruses [1]. A number of these viruses are members of the Japanese encephalitis serogroup. Although there is no known cross-protective immunity among flaviviruses, there is experimental evidence that heterotypic flavivirus antibody can modulate or down-regulate clinical illness and reduce virus loads [1]. Thus, it is possible that the widespread flavivirus antibody among human, domestic animal, and bird populations in tropical America down-regulates or modulates clinical illness and viremia associated with WNV infection and, thus, reduces transmission. A third explanation is that intrinsic or extrinsic factors associated with hosts and the environment may be involved in selecting genetic variants of the virus that are less virulent. Heterotypic flavivirus antibody could influence this selection, as could innate immunity that has evolved as a result of frequent exposure to numerous flaviviruses. Also, ambient temperature, the species of mosquito vectors, secondary vertebrate hosts, and other extrinsic factors could influence selection of less virulent virus strains.
Finally, it is likely that at least some WNV infections are misdiagnosed as dengue infections. Most surveillance for dengue infection in tropical American countries is based on results of the IgM capture ELISA, which is a nonspecific test for flavivirus IgM antibody. Cross-reaction with different flavivirus antigens depends on the infecting virus. In primary infections with dengue viruses, antibody-positive reactions are common with WNV, St. Louis encephalitis virus, Japanese encephalitis virus, and yellow fever virus antigens [27]. In primary infections with these latter viruses, however, the antibody reaction is fairly specific, with only limited cross-reaction with dengue and other flavivirus antigens. On the other hand, in secondary infections with all of the mosquito-borne flaviviruses tested, there is extensive cross-reactivity regardless of the infecting virus [1, 27]. Thus, many WNV infections, especially those in persons who have had a previous heterotypic flavivirus infection, will yield positive results with the dengue IgM ELISA and, thus, will be diagnosed as dengue infections and not WNV infections.
Why Has Wnv Spread so Rapidly in the Past 10 Years?
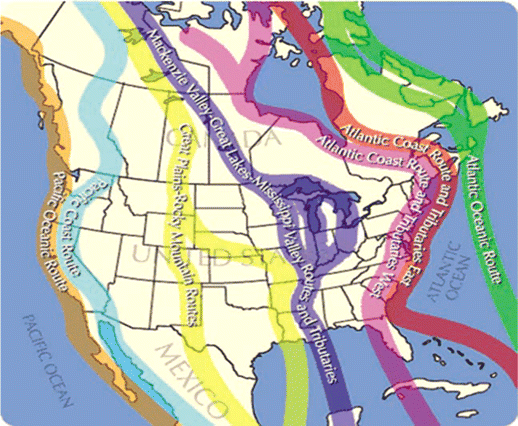
The westward movement of WNV in the United States and Canada can best be explained by introduction via migratory birds that fly south to Central and South America in the fall and back north to the United States and Canada along specific flyways in the spring. Thus, the yearly movement westward in 2001, 2002, 2003, and 2004 shows very good correlation with the Atlantic, Mississippi, Central, and Pacific flyways of migratory birds (figures 1 and 2). The virus caused major epidemics of infection in each of these regions 1 year after introduction via migratory birds, most likely after local dispersion via resident birds.
Migratory bird flyways in the Western Hemisphere. In the fall, birds fly south to areas in tropical America, where they spend the winter. In the spring, they fly north again, potentially carrying the virus with them each way. In both the south and the north, birds from different flyways may mix.
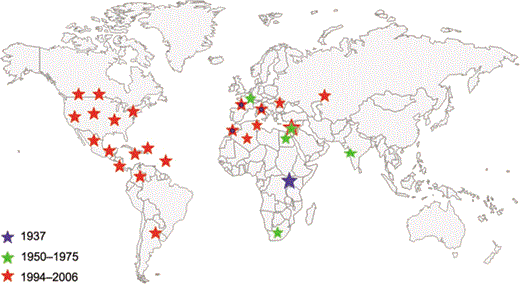
Another mechanism that likely facilitated the spread of the epidemic WNV in both the Old World and the New World is the emergence of a virus strain with greater epidemic potential and virulence. Epidemiologic evidence suggests that such a strain of WNV emerged in North Africa in 1994, when an epidemic and/or epizootic of serologically confirmed WNV infection occurred in Algeria; of 50 cases of neurologic disease, 20 (40%) were diagnosed as encephalitis, and 8 patients (16%) died [6]. In 1996, an epizootic occurred among equines in Morocco, with 94 cases, 42 (45%) of which were fatal; there was 1 human case of encephalitis. That same year, a large epidemic of WNV infection occurred in Bucharest, Romania, in which 835 patients were hospitalized. Samples were obtained from 509 patients, and 393 (77%) of those samples tested positive for WNV antibodies [6, 28]. There were 47 cases of encephalitis (12%) and 17 deaths (4%). In 1997, an epidemic in Tunisia resulted in 173 cases of neurologic disease, with 8 deaths (5%). At approximately the same time, a large epizootic occurred on a commercial goose farm in Israel; unpublished reports indicate that several thousand geese died. In 1998, Italy experienced an epizootic of WNV infection among equines in the Tuscany region [29]; there were 14 cases and 6 deaths. Subsequent serologic studies revealed that ∼40% of horses in the region had WNV neutralizing antibody. In 1998, Israel again had epizootics that involved commercial goose flocks and equines. In 1999, continued epizootic transmission occurred among Israeli geese, and confirmed human transmission occurred in Tel Aviv [15]. The virus caused a large epidemic in Volgograd, Russia [30], and jumped the Atlantic, causing the epidemic in Queens, New York (figure 3) [13].
Epidemics caused by West Nile virus, 1937–2006. The red stars indicate epidemics that have occurred since 1994 that have been associated with severe and fatal neurologic disease in humans, birds, and/or equines.
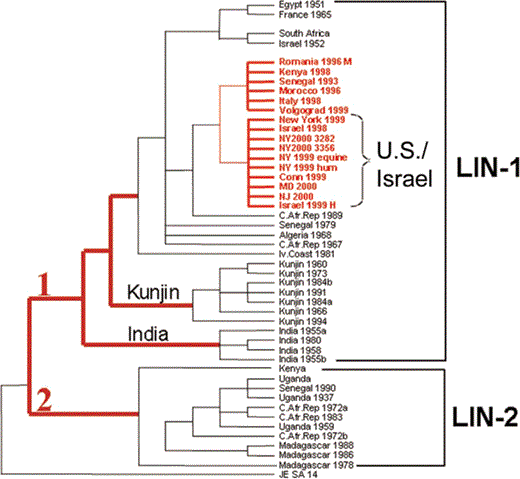
All of these epidemics and epizootics were unique from those that occurred in the past because they were associated with significant severe and fatal neurologic disease in humans, equines, and birds or, in New York, all 3 populations. of interest, before the New York epidemic, few of the epidemics and epizootics had been reported. The epidemiologic and clinical data thus suggest that a virus with greater virulence and epidemic potential emerged that most likely had better fitness and that yielded higher virus loads in susceptible hosts, allowing it to take advantage of modern transportation and globalization to spread in the Mediterranean region, to Europe, and then to the Western Hemisphere. This speculation is supported by sequence data documenting that all of the viruses isolated from these recent epidemics and/or epizootics are closely related genetically: all belong to the same clade and share ⩾98% homology with each other (figure 4) [12, 31], thus suggesting that they all had a common ancestor. Moreover, experimental infections of birds have documented that viruses in this clade, represented by the New York 1999 isolate, have greater virulence than virus strains isolated earlier [32, 33]. An apparent similar change in viral virulence has occurred at least once in the past. In 1962, an epizootic occurred among horses in the Camargue region of France, with a case-fatality rate of 25%–30% [10, 34]. Several human cases of encephalitis were also reported, but WNV was not confirmed to be the etiologic agent. However, WNV was isolated from mosquitoes and humans in the same area in 1964 [34]. It is likely that similar incidents occurred on other occasions but were not detected because surveillance was inadequate or were not reported because the outbreak was localized and did not spread. In the 1990s, the new strain of WNV clearly took advantage of the increased movement of humans and animals resulting from modern transportation and globalization to facilitate geographic spread.
Phylogenetic tree of West Nile viruses based on sequence of the envelope gene. Viruses were isolated during the epidemics indicated by red stars in figure 3, all of which belong to the same clade, suggesting a common origin. Figure appears courtesy of the Centers for Disease Control and Prevention.
The broad vertebrate host and vector range of WNV was another important factor in the successful spread of epidemic/epizootic WNV transmission. The virus has been isolated from 61 species of mosquitoes, >300 species of birds, and >30 species of nonavian hosts since it entered the United States in 1999 (Centers for Disease Control and Prevention; unpublished data). The nonavian vertebrate hosts include rodents, bats, canines, felines, ungulates, and reptiles, in addition to equines and humans (table 1). It is unknown what role any of these species play in the transmission cycle of WNV, but the fact that so many mammal and opportunistic feeding mosquitoes have been found to be infected (table 2) suggests that there may be secondary transmission cycles involving mammals and mammal-feeding mosquitoes. These secondary cycles would put humans and domestic animals at higher risk of infection.
Nonavian species affected by West Nile virus in the United States, 1999–2003.
Host-feeding patterns of selected West Nile virus–positive mosquito species.
Prospects for the Future
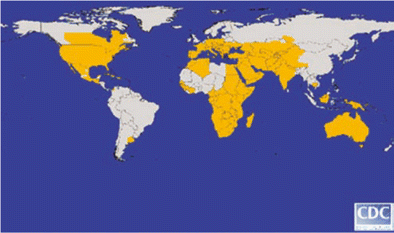
In the 8 years since its introduction into the Western Hemisphere, WNV has established enzootic transmission cycles in nearly every part of the United States and southern Canada. The fact that transmission has also been documented in the Caribbean and in Central and South America makes it likely that WNV has wider distribution in that region than has been reported. It is still uncertain what kind of transmission pattern WNV will ultimately develop in the New World, but the pattern to date suggests that the temperate regions of its geographic range will experience epizootic/epidemic transmission on a regular basis for an indefinite period, whereas the tropical regions will experience sporadic enzootic transmission in a pattern similar to the patterns for Japanese encephalitis virus and St. Louis encephalitis virus. For reasons that are not fully understood, the virus is not causing severe and fatal neurologic disease in tropical regions of the America region as it has in the United States and Canada. The recent detection of several cases of WNV-associated meningoencephalitis in humans in Argentina, however, suggests that the temperate region of the South American Hemisphere is also at risk for severe and fatal disease (Delia Ernia; personal communication). Regardless of clinical presentations, however, it seems clear that WNV has become firmly established in much of the Western Hemisphere, making it one of the most widely distributed of the flaviviruses (figure 5).
The approximate global distribution of West Nile virus, by country, state, and province, 2006. Figure appears courtesy of the Centers for Disease Control and Prevention.
An important question that remains to be answered is whether WNV will continue its westward movement and establish transmission in Hawaii and other islands of the Pacific. The introduction of this virus to Hawaii would have important public health, wildlife, and economic impacts on the state. Hawaii is one of the few pristine tropical environments left in the world where there is no major risk for infection with vectorborne diseases. Enzootic/epizootic WNV transmission would not only affect the public health of the people of Hawaii, but it could have a significant, negative impact on the tourism industry and, thus, on the economy of Hawaii in general. Moreover, the introduction of WNV to the islands could possibly push a number of threatened native bird species to the brink of extinction [35].
There are several potential ways that WNV could be introduced to Hawaii, including (1) via infected humans, mosquitoes, birds, or other vertebrate hosts arriving via airplane and ship from the mainland United States; (2) via migratory birds; or (3) via vaccination of equines and other animals. The first option is probably the greatest threat, but migratory birds do fly from the West Coast of North America to Hawaii. The third option is remote but possible, because it is well known that, in rare instances, killed vaccines fail, and vaccinated animals develop an acute infection with the agent that they were vaccinated against. Culex quinguefasciatus and Aedes albopictus, 2 mosquito vectors of WNV on the mainland, are widespread in Hawaii.
At present, there is little chance that WNV will be eliminated from the Western Hemisphere. Its broad host and vector range ensures that it will persist in many different ecologic systems for the indefinite future. Although surveillance for WNV and other arboviruses has improved greatly in the United States and Canada in the past 8 years, unfortunately, it is still not good enough to predict where epidemic/epizootic transmission will occur. Surveillance in tropical America is complicated by the presence of a number of other enzootic/endemic flaviviruses and by the general lack of adequate laboratory capacity to distinguish among them. Moreover, mosquito-control programs lack adequate personnel, equipment, and tools in most of the region.
There are a number of vaccines being developed for WNV infection, some of which are already being effectively used to prevent equine infection. A major dilemma for human use, however, is to identify the target population, such that vaccination will be cost-effective [36, 37]. Currently, vaccination would have to target persons aged >50 years in all areas of the United States and Canada, where there is a risk for recurrent epizootic/epidemic transmission.
In conclusion, WNV transmission will be very difficult to prevent and control because of the poor public health infrastructure to deal with vectorborne and zoonotic diseases. The cost-effectiveness of human vaccines is uncertain. Improvement of the laboratory diagnostic, epidemiologic, and vector control infrastructure will be critical for the development of effective prevention and control strategies for WNV infection, as well as for other potential emerging vectorborne viral diseases, such as urban yellow fever and Rift Valley fever, both of which have the potential to spread rapidly in the region if the opportunity arises.
acknowledgments
Potential conflicts of interest. D.J.G.: no conflicts.







Comments