-
PDF
- Split View
-
Views
-
Cite
Cite
Marcus D. Flather, Marcelo C. Shibata, Andrew J.S. Coats, Dirk J. Van Veldhuisen, Aleksandr Parkhomenko, Joszef Borbola, Alain Cohen-Solal, Dan Dumitrascu, Roberto Ferrari, Philippe Lechat, Jordi Soler-Soler, Luigi Tavazzi, Lenka Spinarova, Jiri Toman, Michael Böhm, Stefan D. Anker, Simon G. Thompson, Philip A. Poole-Wilson, Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS), European Heart Journal, Volume 26, Issue 3, February 2005, Pages 215–225, https://doi.org/10.1093/eurheartj/ehi115
Close - Share Icon Share
Aims Large randomized trials have shown that beta-blockers reduce mortality and hospital admissions in patients with heart failure. The effects of beta-blockers in elderly patients with a broad range of left ventricular ejection fraction are uncertain. The SENIORS study was performed to assess effects of the beta-blocker, nebivolol, in patients ≥70 years, regardless of ejection fraction.
Methods and results We randomly assigned 2128 patients aged ≥70 years with a history of heart failure (hospital admission for heart failure within the previous year or known ejection fraction ≤35%), 1067 to nebivolol (titrated from 1.25 mg once daily to 10 mg once daily), and 1061 to placebo. The primary outcome was a composite of all cause mortality or cardiovascular hospital admission (time to first event). Analysis was by intention to treat. Mean duration of follow-up was 21 months. Mean age was 76 years (SD 4.7), 37% were female, mean ejection fraction was 36% (with 35% having ejection fraction >35%), and 68% had a prior history of coronary heart disease. The mean maintenance dose of nebivolol was 7.7 mg and of placebo 8.5 mg. The primary outcome occurred in 332 patients (31.1%) on nebivolol compared with 375 (35.3%) on placebo [hazard ratio (HR) 0.86, 95% CI 0.74–0.99; P=0.039]. There was no significant influence of age, gender, or ejection fraction on the effect of nebivolol on the primary outcome. Death (all causes) occurred in 169 (15.8%) on nebivolol and 192 (18.1%) on placebo (HR 0.88, 95% CI 0.71–1.08; P=0.21).
Conclusion Nebivolol, a beta-blocker with vasodilating properties, is an effective and well-tolerated treatment for heart failure in the elderly.
See page 203 for the editorial comment on this article (doi:10.1093/eurheartj/ehi118)
Introduction
Heart failure is a common syndrome with disabling symptoms and a poor prognosis. In Europe, 1% of persons are affected, with both incidence and prevalence increasing sharply with age.1–3 The condition accounts for 2% of all healthcare spending.4 Heart failure is usually associated with impaired systolic ventricular function (heart failure with a large heart and decreased ejection fraction) but may also present with preserved systolic ventricular function (normal sized heart and near-normal ejection fraction).5 This latter entity is more common in the elderly.6
Heart failure is characterized by changes in many neurohormonal mechanisms, but notably activation of the sympathetic and renin–angiotensin–aldosterone systems. Inhibition of these two systems is the mainstay of current treatment.7 Beta-blockers have been shown to reduce adrenergic drive, improve autonomic balance, and reduce ventricular wall stress.8,9 Several large randomized trials and meta-analyses have shown that in patients with a low ejection fraction, beta-blockers reduce hospital admissions for worsening heart failure and the risk of death by ∼30%.10–14
The average age of patients in these previous placebo-controlled mortality and morbidity studies of beta-blockers in heart failure has been 63 years, and patients with an ejection fraction >40% were excluded.14 In a community-based study, the average age of new incident cases of heart failure was 76 years.3 The lack of evidence from a representative sample of elderly patients with heart failure has raised doubts about extrapolating the current evidence for beta-blockers to patients in the community. More relevant evidence from randomized trials is needed to clarify the balance of risk and benefit of beta-blockers in elderly patients with a broad range of ventricular function.
Nebivolol is a beta-1-selective blocker with vasodilating properties related to nitric oxide modulation.15–17 This effect may improve tolerability in elderly patients with heart failure, where endothelial vasodilator reserve may be limited. There is also evidence that nebivolol may have advantages in patients with diastolic dysfunction.18 The SENIORS Study (Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisation in Seniors with Heart Failure) was undertaken to determine the effect of nebivolol on mortality and morbidity in elderly patients with heart failure, regardless of ejection fraction.
Methods
Study design
The protocol for SENIORS has been published elsewhere.19 SENIORS is a parallel group, randomized, double-blind, multi-centre, international trial comparing nebivolol with placebo in elderly patients with heart failure on optimal standard therapy. The study was performed in compliance with good clinical practice and followed the recommendations of the Declaration of Helsinki. The relevant national and local ethics review boards and regulatory authorities approved the protocol. Written informed consent was obtained from all patients before enrolment.
Patients
To be eligible, patients had to be aged ≥70 years, provide written informed consent, have a clinical history of chronic heart failure with at least one of the following features: documented hospital admission within the previous 12 months with a discharge diagnosis of congestive heart failure or documented left ventricular ejection fraction ≤35% within the previous 6 months. The main exclusion criteria were new drug therapy for heart failure in the 6 weeks prior to randomization, any change in cardiovascular drug therapy in the 2 weeks prior to randomization, heart failure due primarily to uncorrected valvular heart disease, contraindication or previous intolerance to beta-blockers (e.g. heart rate <60 beats/min or systolic blood pressure <90 mmHg), current use of beta-blockers, significant hepatic or renal dysfunction, cerebrovascular accidents within the previous 3 months, and being on a waiting list for percutaneous coronary intervention or cardiac surgery or other major medical conditions that may have reduced survival during the period of the study.
Screening and randomization
Patients were screened for eligibility at participating centres by checking hospital outpatient lists and admissions for heart failure within the previous year. Patients underwent echocardiography after entry into the study but prior to administration of the study drug. A master randomization list stratified by centre was prepared and held securely. Randomization to nebivolol or placebo on a 1:1 basis was carried out by telephone call to a central office (Clinical Data Care, Lund, Sweden). Patients were allocated a treatment number which corresponded to the appropriate study treatment packs.
Study medication
Nebivolol or placebo tablets were provided in identical packaging and tablet appearance. The initial dose was 1.25 mg once daily, and, if tolerated, this was increased to 2.5 and 5 mg, respectively, every 1–2 weeks, reaching a target of 10 mg once daily over a maximum of 16 weeks. Dose titration was performed during a visit to the hospital or clinic, and patients were observed for up to 2 h after taking the new dose to assess tolerability. Up-titration could be stopped or delayed depending on symptoms, possible side-effects, or at the judgement of the local investigator.
Patient follow-up
Following the titration phase, the next two visits were scheduled at 4 and 6 months after randomization, and further visits at 3-monthly intervals until the end of the study. A 30-day safety follow-up visit was planned for all patients after the last study drug administration to document any post-treatment adverse effects. The initial protocol specified a minimum observation period of 6 months for all patients, but this was amended to a minimum of 12 months by the Steering Committee in March 2003 when it was observed in blinded analysis that the composite primary event rate was below the expected rate. The end of the observation period for all patients was set at 15 November 2003. In those patients who did not attend their end of observation follow-up visit, vital status was determined by direct enquiry, national registers of death, and review of practice records.
Monitoring, data management, and reporting of adverse events
Study monitoring and data management were performed by the contract research organization Parexel GmbH (Berlin, Germany) under the supervision of the Sponsor. Patient data were recorded on paper case report forms. At regular intervals validation of data was undertaken and all resulting queries were resolved with investigators. Serious adverse events, other than those defined as clinical outcome events, were reported by investigators in an expedited manner and reviewed centrally. An independent and experienced Data and Safety Monitoring Committee regularly examined unblinded data prepared by an independent statistician. In all four interim unblinded analyses,19 no reason was found for safety concerns.
Independent supervision of the study
The protocol was developed by an independent Steering Committee in collaboration with the Sponsor. The Steering Committee comprised cardiologists from hospitals and academic centres in the participating countries. The responsibilities of the Steering Committee included overseeing the scientific conduct of the study, independent statistical analysis, presentation of the results at scientific meetings, and preparation of manuscripts for publication. The independent Data and Safety Monitoring Committee was responsible for reviewing unblinded study data at regular intervals and ensuring that the Steering Committee was informed of any safety concerns or efficacy issues during the course of the study. All reported deaths and hospital admissions were referred to the independent Clinical Events Review Committee, blinded to treatment, for review of medical records and any other documentation and for final classification of events.
Study outcomes and definitions
The primary outcome was the composite of all cause mortality or cardiovascular hospital admission (time to first event). This outcome was chosen to reflect the potential effect of nebivolol on quality of life in elderly patients in addition to an effect on the risk of death. Secondary outcomes included all cause mortality, the composite of all cause mortality or all cause hospital admissions, all cause hospital admissions, cardiovascular hospital admissions, cardiovascular mortality, the composite of cardiovascular mortality or cardiovascular hospital admissions (time to first event for all of them), functional capacity by New York Heart Association (NYHA) class assessment, and 6-min walk test at 6 months. Deaths were sub-classified by the Clinical Event Review Committee into cardiovascular deaths, non-cardiovascular deaths, or deaths of unknown cause. Hospital admissions were defined as admissions to hospital involving a stay of at least 24 h (excluding hospital admissions planned before randomization and planned admissions for study-related procedures). Cardiovascular admissions included those for cardiac causes (e.g. worsening heart failure, acute coronary syndromes), cerebrovascular causes, or other cardiovascular causes. All other causes of hospital admission were classified as non-cardiovascular or unknown, if relevant information was missing.
Statistical methods
On the basis of previous studies, it was estimated that a sample size of 1700 patients observed for an average 1.5 years (2 years for recruitment and a minimum follow-up of 0.5 years) would provide 90% power to detect a proportional difference of 25% between nebivolol and placebo with a two-sided significance level of 0.05. This assumed a mean annual rate for the primary outcome of 25% in the placebo group and an overall non-compliance rate of 20% in the nebivolol group. To allow for the possibility of lower than expected event rates and higher levels of non-compliance, and losses to follow-up, a total sample of 2000 patients was specified. The sample size was estimated using a time-driven rather than an event-driven approach. The statistical analysis was carried out using the intention to treat according to a plan drawn up before the outcome data were available. The pre-specified primary outcome was analysed using a Cox proportional hazards model with randomized treatment as the major covariate adjusted for baseline age, gender, and ejection fraction. A further analysis was performed with treatment as the sole covariate. Similar analyses were carried out for secondary outcomes unadjusted for multiple comparisons. Whether the effect of nebivolol varied according to five pre-specified risk factors of major interest (Figure 4) was assessed by tests of interaction in Cox-proportional hazard regression analyses adjusted for age, gender, and ejection fraction. A two-sided significance level of 0.05 was specified. Seven exploratory analyses that were not pre-specified in the statistical analysis plan are reported and are clearly labelled as such.
Results
Enrolment and baseline characteristics
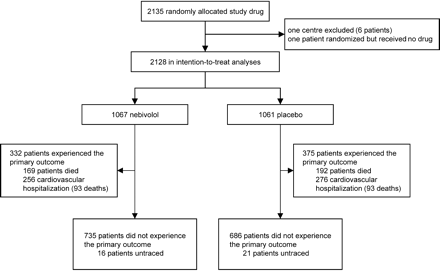
The first patient was enrolled in September 2000, the last patient in December 2002. The date of study end was specified as 15 November 2003 for all patients. A total of 2135 patients were enrolled from 11 countries: Czech Republic (312 patients), France (62), Germany (56), Italy (54), Hungary (175), The Netherlands (341), Romania (370), Spain (137), Switzerland (18), Ukraine (457), and the United Kingdom (153). Seven patients were excluded from the final analysis (all six patients recruited at one centre after an audit showed significant deviations from the protocol and poor data quality, and one patient who never took study medication after randomization). A total of 2128 patients (1067 in the nebivolol group and 1061 in the placebo group) were available for analysis (Figure 1).
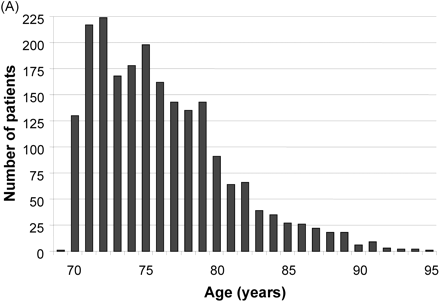
Patient characteristics at baseline are provided in Table 1. There were no differences among the randomized groups in any of the important baseline characteristics. Mean duration of patient follow-up was 21 months (SD 9; inter-quartile range 14–29 months) with 1863 patient-years of follow-up in the nebivolol group and 1839 in placebo (Table 2). The distribution of patients by age and ejection fraction are shown in Figure 2A and B, respectively.
Compliance to treatment
The mean maintenance dose in the nebivolol group was 7.7 mg (SD 3.6) and 8.5 mg (SD 3.1) in the placebo group. The proportion of patients reaching a dose of ≥5 mg at the end of the titration phase was 80% in the nebivolol group compared with 87% in placebo, and the proportions reaching 10 mg were 68 and 80%, respectively. Premature discontinuation for any reason other than death occurred in 27 and 25%, respectively (Table 2). Discontinuation of treatment occurred mostly at the patients' request or for other non-clinical reasons, with little difference between the two groups (Table 2).
Haemodynamics
Mean (SD) systolic blood pressure, diastolic blood pressure, and heart rate at the first maintenance visit (∼4 months), taking the last observation available for patients with missing data, were 132.3 (19.9) and 135.2 (20.3) mmHg, 76.3 (10.4) and 78.1 (10.2) mmHg, and 68.8 (12.5) and 77.4 (13.6) beats/min in the nebivolol and placebo groups, respectively. Changes from baseline [mean (SD)] in the nebivolol and placebo groups, respectively, for systolic blood pressure were −6.3 (18.2) and −4.3 (17.9) mmHg, for diastolic blood pressure −4.2 (10.6) and −2.4 (10.0) mmHg, and for heart rate −10.3 (14.3) and −1.5 (14.0) beats/min.
Primary outcome
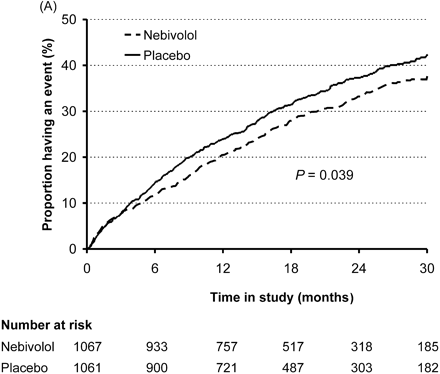
The proportion of patients who suffered death or cardiovascular hospital admission in the nebivolol group was 31.1% compared with 35.3% in the placebo group [hazard ratio (HR) 0.86, 95% CI 0.74–0.99; P=0.039; Table 3]. The unadjusted analysis showed an HR of 0.85 with the same CI (P=0.034; Table 3). The absolute risk reduction is 4.2%, suggesting that 24 patients would need to be treated for 21 months to avoid one event. The Kaplan–Meier plot (time to event) suggests that the curves for nebivolol-treated patients compared with placebo for the primary outcome separate after about 6 months and continue to diverge for the duration of follow-up (Figure 3A).
Primary outcome in subgroups
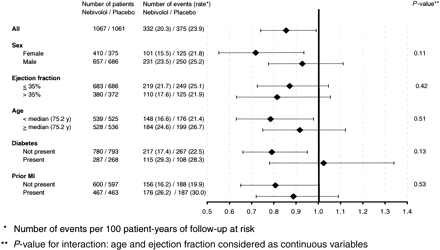
Results for the primary outcome stratified by major clinical subgroups including gender, ejection fraction, age, diabetes, and prior myocardial infarction are shown in Figure 4. There was no statistically convincing evidence that any of these factors modified the effect of nebivolol. The HR for patients aged less than the median of 75.2 years was 0.79 (95% CI 0.63–0.98) and 0.92 (95% CI 0.75–1.12) in those aged >75.2 years (for interaction test P=0.51; Figure 4). For men, the HR was 0.93 (95% CI 0.78–1.11) and for women, 0.72 (95% CI 0.55–0.93) (for interaction test P=0.11; Figure 4). The HR for patients with left ventricular ejection fraction ≤35 and >35% were 0.87 (95% CI 0.73–1.05) and 0.82 (95% CI 0.63–1.05), respectively (for interaction test P=0.42; Figure 4).
All cause mortality
The proportion of deaths was 15.8 and 18.1% in the nebivolol and placebo groups, respectively (HR 0.88, 95% CI 0.71–1.08; P=0.21; Table 3). Time to all cause death is shown in Figure 3B. The proportion of cardiovascular deaths which might be considered sudden deaths was 36% in the nebivolol group and 48% in the placebo group.
Other secondary outcomes
The proportions of cardiovascular hospital admissions were 24.0 and 26.0% in the nebivolol and placebo groups, respectively (HR 0.90, 95% CI 0.76–1.06). Other secondary outcomes including total mortality, cardiovascular mortality, or all cause mortality and all cause hospitalization are shown in Table 3.
Analyses which were not pre-specified
In order to put the results of SENIORS in the context of previous beta-blocker trials, which recruited younger patients and excluded patients with higher ejection fractions, we identified the subgroup of patients most similar to the previous major outcome trials. In this subgroup, defined as patients of less than median age (75.2 years) with an ejection fraction ≤ 35% (n=342 for nebivolol and n=342 for placebo), the HR for the primary outcome was 0.73 (95% CI 0.56–0.96). For all cause mortality alone, the HR was 0.62 (95% CI 0.43–0.89). This suggests an efficacy for nebivolol similar to that seen in similar patient cohorts for metoprolol-controlled release, bisoprolol, and carvedilol. If an ejection fraction threshold of 40% rather than 35% was used, there was no material difference to the results for the subgroup comparisons. For the primary endpoint, in the population with ejection fraction ≤40%, HR=0.86 (95% CI 0.73–1.03; P=0.095) and for ejection fraction >40%, HR=0.83 (95% CI 0.62–1.11; P=0.203). As age was a particular focus of the SENIORS trial, we also analysed patient cohorts between median age (75.2 years) and 85 years (n=459 for nebivolol and n=482 for placebo) where the HR for the primary endpoint was 0.91 (95% CI 0.74–1.13) and for patients >85 years (n=69 for nebivolol and n=54 for placebo) where the HR was 1.32 (95% CI 0.73–2.37). There was no difference between the groups for hospitalization for heart failure [placebo 144 (13.7%), nebivolol 145 (13.9%), HR=0.99 (95% CI 0.79–1.25; P=0.95)].
Adverse events
Adverse events are shown in Table 4. There were no differences other than those expected of a beta-blocker, namely, an increased incidence of bradycardia and a decrease of angina pectoris and unstable angina. Bradycardia was associated with withdrawal from blinded treatment in 18 and 4 nebivolol and placebo treated patients, respectively.
Discussion
The SENIORS study shows that treating elderly patients with heart failure with nebivolol reduces the composite risk of all cause mortality or cardiovascular hospital admission compared with placebo. The beneficial effects appear after 6 months of treatment and the risk reduction continues to increase with longer treatment. A broad range of patients on standard currently recommended treatment were included (one-third with ejection fraction >35%, 37% females, and 50% aged ≥75 years). Study medication was well tolerated and the majority of patients were able to reach a maintenance dose of 10 mg once daily after careful titration. The observed reductions in heart rate and blood pressure were as anticipated from treatment with a beta-blocker.
Several large randomized trials and meta-analyses of previous trials have shown that beta-blockers reduce the risk of death or hospitalization in patients with heart failure.10–14 In those studies the mean age was 63 years and the estimated mean ejection fraction was 25% (most studies had an upper limit for ejection fraction of 35 or 40%). Subgroup analyses of some of the large studies indicated this benefit was present in patients>65 years,13,20,21 but there was little information in those >70 years and those patients with ejection fraction >35%. The need for additional data has been emphasized.8,11,22,23 SENIORS extends the evidence of benefit of beta-blockade to a broad range of elderly patients with heart failure including those with mild left ventricular dysfunction or preserved ventricular function. The patients in the SENIORS study more closely resemble the general population of patients with heart failure, where the mean age is 76 years.2,3 Other observational studies have assessed the effects of beta-blockers in elderly patients24,25 but no previous randomized controlled trial has had the power to demonstrate efficacy in elderly heart failure patients specifically.
The estimated HR for the primary outcome in SENIORS was 0.85, suggesting a lesser degree of risk reduction compared with previous large trials. Nevertheless, both components of the primary outcome (all cause mortality and cardiovascular hospital admission) show a similar and consistent effect indicating that the results are robust with a 15% proportional and 4% absolute reduction in risk. We undertook a subanalysis which was not pre-specified (age less than median and ejection fraction ≤35%) in order to determine the result for patients most similar to those recruited in previous trials. The findings were similar to the results from previous trials, with significant 27 and 38% reductions in the primary composite endpoint and all cause mortality, respectively, indicating that nebivolol has beneficial effects of a magnitude similar to those of other beta-blockers proved to have major outcome benefits in heart failure.
SENIORS explored the effects of nebivolol in several clinically important subgroups of patients. Given the overall P-value for the primary endpoint of 0.039 there was, of course, limited power to detect potential interactions. Despite advanced age being an inclusion criterion one important potential interaction was still that of age itself. The increased risk of dying from a natural cause in the elderly may compete with the potential benefits of a treatment. Thus, it is plausible that there is a threshold of biological age, rather than chronological age, beyond which the benefit of treatment is difficult to show. Although the benefits of nebivolol appeared to be less in patients >75 years, age as a continuous variable did not significantly affect the treatment effect. It is possible that beta-blockers, or indeed any treatments, are in reality less effective in the very elderly.
Heart failure with preserved systolic function is common in population samples of heart failure patients, especially in the older age groups.26 Previous mortality and morbidity studies both for ACE inhibitors and for beta-blockers have typically excluded such patients. One trial within the CHARM programme27 investigating the effects of candesartan did not show a significant effect on its primary endpoint. SENIORS showed both a significant overall treatment effect and a virtually identical point estimate of risk reduction for patients with low and preserved ejection fraction. This is the best evidence to date of a treatment likely to be effective in the substantial proportion of the elderly population with heart failure who have a broad range of ventricular dysfunction, and suggests the beta-blocker nebivolol can be recommended for heart failure, irrespective of ejection fraction.
Nebivolol is a beta-1-selective blocker with vasodilator properties which appear to be due to modulation of nitric oxide release that reduces peripheral vascular resistance.15–17 Nebivolol has been extensively investigated in hypertension and has received approval in many jursidictions for this indication. The exact mechanisms of benefit in heart failure are not known, but may include reduction in ventricular wall stress,28 reduction in adverse neurohormonal stimulation, and reduction in incidence of acute coronary events, especially as half of the patients had a prior myocardial infarction. In SENIORS, patients were started on a dose of 1.25 mg once daily and titrated to a target dose of 10 mg over a mean of 7 weeks. Elderly patients are at higher risk of hypotension, bradycardia, and other side-effects of beta-blockers when compared with younger patients. Nevertheless, 68% of patients in the nebivolol group reached the maximum dose, with only 6% not tolerating any dose. This good tolerability may, in part, be related to the vasodilating properties of nebivolol so that results may not be generalizable to other beta-blockers when used in elderly heart failure patients. Previous studies have raised the possibility that beta-blockers in heart failure need to be evaluated separately and the effects of one cannot be extrapolated to another.29,30 It has been estimated that the probability of receiving beta-blocker therapy is substantially lower in the elderly31,32 and the most quoted reasons for this are concerns that physicians are less convinced by the body of evidence in this age group.33,34 The SENIORS study should allay this concern.
The SENIORS trial shows that the beta-1-selective vasodilating beta-blocker nebivolol is well tolerated and effective in reducing mortality and morbidity in patients of age >70 years with heart failure, regardless of the initial ejection fraction. Nebivolol can be used in this common group of patients for the treatment of heart failure.
Acknowledgements
We thank all those patients who made this study possible by agreeing to participate. We thank investigators, study nurses, and co-ordinators for their work in completing the study. The study was supported by Menarini Ricerche SpA. Authors were funded to attend meetings and to undertake work related to the trial. M.D.F. received a grant for secretarial services to the Steering Committee. S.T. was reimbursed by Menarini Ricerche SpA for membership of the Data Safety and Monitoring Committee and for acting as an independent statistical advisor to the Steering Committee. All members of the Steering Committee have received honoraria for speaking on aspects of heart failure at meetings funded by companies in the pharmaceutical industry. All have participated in or are involved in other studies or research projects funded by industry.
Appendix
Steering Committee
Philip Poole-Wilson (co-chair) Imperial College of Science Technology and Medicine and Royal Brompton and Harefield NHS Trust, London, UK; Andrew Coats (co-chair) Faculty of Medicine, University of Sydney, Sydney, Australia; Stefan Anker, Department of Cardiology, Charité Campus Virchow-Klinikum, Berlin, Germany; Michael Böhm, Universitätskliniken des Saarlandes Innere Medizin III, Homburg/Saar, Germany; Joszef Borbola, Hungarian Institute of Cardiology, Budapest, Hungary; Alain Cohen-Solal, Hopital Beaujon, Paris, France; Dan Dumitrascu, University of Pharmacy and Medicine, Cluj Napoca, Romania; Roberto Ferrari, University of Ferrara, Ferrara, Italy, and Salvatore Maugeri Foundation, IRCCS, Gussago, Italy; Marcus Flather, Imperial College of Science Technology and Medicine and Royal Brompton and Harefield NHS Trust, London, UK; Philippe Lechat, Hopital Pitie-Salpetriere, Paris, France; Aleksandr Parkhomenko, Institute of Cardiology, Academy of Science of Ukraine, Kiev, Ukraine, Marcelo Shibata, Division of Cardiology, University of Alberta, Edmonton, Canada; Jordi Soler-Soler, Hospital General Vall d'Hebron, Barcelona, Spain; Luigi Tavazzi, IRCCS Policlinico San Matteo, Pavia, Italy; Jiri Toman (deceased); Lenka Spinarova, Masaryk University Hospital, Brno, Czech Republic; Dirk Van Veldhuisen, University Hospital Groningen, The Netherlands. (P.P.-W., A.C., M.F., A.P., M.S., and D.V.V. are members of the writing committee. All contributors have seen and approved the final version of this manuscript.)
Data Safety and Monitoring Committee
Alberto Zanchetti (chair), Università di Milano and Instituto Auxologico, Italy; Basil Lewis, Lady Davis Carmel Medical Center, Haifa, Israel; Markku Nieminen, Helsinki University Central Hospital, Helsinki, Finland; Peter Sleight, University of Oxford, Oxford, UK; Simon Thompson, Medical Research Council Biostatistics Unit, Cambridge, UK. Independent statistical analyses for the Data Safety and Monitoring Committee and for the manuscript were provided by Tony Brady (Sealed Envelope Ltd, London, UK). (S.T. has overseen the data analysis and approved the final version of this manuscript.)
Clinical Event Review Committee
Kristian Thygesen (chair), Aarhus University Hospital, Denmark; Michael Frenneaux, University of Wales, UK; Michal Tendera, Silesian School of Medicine, Katowice, Poland; Marcelo Shibata (representative of the Steering Committee), Division of Cardiology, University of Alberta, Edmonton, Canada; Gianfranco Sinagra, Ospedale Maggiore, Trieste, Italy.
Study Co-ordination
Menarini Ricerche SpA (study no. MR/01-99/01-Nhf): Angela Capriati (corporate clinical research director), Ines Koch (study manager), Kai Schumacher (study physician), Giacomo Mordenti (statistician), Maria Mercede Brunetti (quality assurance), Isabel Paredes (drug safety unit)
Parexel GmbH: Stephan Jung (senior project manager), Barbara Veprek (project manager), Werner Wierich (senior director biostatistics and programming)
Clinical Trials and Evaluation Unit (Secretariat to Steering Committee): Marcelo Shibata, Nicola Delahunty, Anil Taneja, Marcus Flather, Jean Booth
Investigators
Czech Republic: Prof. Michael Aschermann, MUDr Jiri Carda, MUDr Bohuslav Cernosek, MUDr Jaroslav Doubravský, Prim. MUDr Bohumil Filipenský, Prof. Pavel Gregor, MUDr Petr Kovár, MUDr Josef Kroupa, MUDr Milena Kubicková, MUDr Igor Macel, MUDr Frantisek Padour, MUDr Antonin Pavelka, MUDr Lubomir Pollák, MUDr Jana Popelová, MUDr Karel Pospisil, MUDr Petr Reichert, Prof. Borivoj Semrad, MUDr Hana Skalická, As. MUDr Rudolf Spacek, Doc. MUDr Jindrich Spinar, Prof. Lenka Spinarova, MUDr Dag Tichy, Prof. Jiri Toman, MUDr Petr Vales
France: Prof. Pierre Bareiss, Dr Hassan Belfaqih, Prof. Joel Belmin, Dr Patrick Bocquet, Dr Jean-Patrick Bousser, Dr Francois Funck, Prof. Michel Galinier, Dr Eric Garbarz, Dr Richard Isnard, Prof. Guillaume Jondeau, Dr Jean-Claude Kahn, Dr Khalifé Khalife, Dr Pierre Larrieu, Dr André Marquand, Dr Yannick Neuder, Dr Jean-Francois Thébaut
Germany: Prof. Michael Böhm, PD Dr Uwe Desaga, Prof. Stephan Holmer, Dr Detlev Nunberger, Prof. Burkert Pieske, Dr Andreas Schreckenberg, Dr Walter Sehnert, Prof. Wolfram Voelker, Dr Jürgen Wachter
Hungary: Prof. József Borbola, Dr Béla Herczeg, Prof. Pál Kárpáti, Dr Béla Öze, Dr Béla Pálossy, Dr László Regós, Dr Aladár Rónaszéki, Dr Mátyás Sereg, Dr Sándor Tímár, Dr Csaba Tóth, Dr Károly Tóth, Dr József Ványi, Dr István Varga, Prof. Gabor Veres
Italy: Dr Aldo Carbonari, Dr Giuseppe Castiglioni, Prof. Roberto Ferrari, Dr Antonio Grieco, Dr Francesco Ippolito, Dr Danilo Neglia, Dr Gianfranco Parati, Dr Roberto Pedretti, Dr Gianni Rovelli, Dr Franco Russo, Dr Vito Santoro, Prof. Luigi Tavazzi, Dr Massimo Uguccioni
Netherlands: Dr Adriaan Bredero, Dr Jeroen Bucx, Dr Eugene Buys, Dr P.A.R. de Milliano, Dr Anita Derks, Dr Peter Dunselman, Dr Erwin Göbel, Dr B. Hamer, Dr J.A. Henneman, Dr Walter R.M. Hermans, Dr G.M. Jochemsen, Dr Hans Kragten, Dr T. Gie Liem, Dr G.C.M. Linssen, Dr Angela Maas, Dr M. Nagelsmit, Dr J. Posma, Dr Brian van den Berg, Dr Pieter van der Burgh, Dr Louis Van Kempen, Dr Rudolf A.M. van Langenveld, Prof. Dirk Jan van Veldhuisen, Dr Leen Van Wijk, Dr Jan C.L. Wesdorp, Dr Adrie Withagen
Romania: Prof. Ioan Victor Bruckner, Prof. Alexandru Campeanu, Prof. Radu Capalneanu, Conf. Dr Emilian Carasca, Dr Zorita Ciulea-Prepelita, Dr Mihail Crisu, Dr Sabina Diaconescu, Prof. Maria Dorobantu, Sef lucrari Dan Dumitrascu, Conf. Dr Carmen Ginghina, Prof. Alexandru Ioan, Dr Ferencz Jeszenszky, Dr Dorina Leasu, Conf. Dr Ioan Manitiu, Dr Constanta Motoc-Moarcas, Dr Codin Olariu, Dr Eugen Panturu, Prof. Mariana Radoi, Dr Marcela Rau, Conf. Dr Crina Sinescu, Dr Nicolae Stanciu, Sef lucrari Dr Mihail Stefanescu, Conf. Dr Cristina Tanaseanu, Dr Rodica Tataru, Dr Gabriel Tatu-Chitoiu, Dr Sebastian Toma, Dr Lacramioara Topolnitchi, Dr Alina Tudose, Conf. Dr Marius Vintila
Spain: Dr Fernando Alonso, Dr Julián Bayón, Dr Jordi Bruguera, Dr Román Calvo-Jambrina, Dr Jose M. Cruz-Fernandez, Dr Alfonso del Río, Dr Miguel Angel de Miguel, Dr Enrique Galve, Dr Amador López-Granados, Dr Ramón Martos, Dr Juan Motero, Dr Miguel Ribas, Dr Antonio Salvador, Dr Luis Sosa, Prof. Mariano Valdés, Dr Vicente Valle, Dr José A. Velasco
Switzerland: Prof. Augusto Franco Gallino, Prof. Tiziano Moccetti
Ukraine: Prof. Ekaterina Amosova, Dr Olga Barna, Dr Andrey Basylevych, Dr Nikolay Demeshko, Prof. Aleksandr Djadyk, Dr Boris Goloborodko, Prof. Tatjana Khomazyuk, Dr Sergiy Kolomiets, Prof. Yuriy Koltchin, Acad. Oleg Korkuschko, Prof. Volodymyr Kovalenko, Dr Igor Kovalskiy, Dr Igor Krayz, Prof Volodymyr Kubyshkin, Dr Svetlana Lichkanenko, Prof. Yuriy Mostovoy, Prof. Vasil Netyazhenko, Prof. Aleksandr Parkhomenko, Dr Mikhaylo Perepelyca, Prof. Tatyana Pertseva, Dr Roman Petrovskiy, Prof. Sergiy Polyvoda, Prof. Mykola Rishko, Dr Leonid Rudenko, Dr Yurii Rudyk, Prof. Ivan I. Sakharchuk, Prof. Viktor Syvolap, Dr Susanna Tikchonova, Dr Larisa Vasiljeva, Prof. Nikolay Vatutin, Prof. Vadim Vizir, Dr Andreiy Yahenskiy, Dr Anatoliy Zavgorodniy, Dr Vladymir Zhelezniy, Dr Alexey Zhumro
United Kingdom: Dr G.B. Ambepitiya, Dr Allan Bridges, Dr Kevin Channer, Dr Piers Clifford, Prof. Andrew Coats, Dr I.N. Findlay, Dr Robert Greenbaum, Dr Derek Harrington, Dr Sarfraz R. Khan, Prof. Gregory Lip, Dr Donald MacLeod, Dr Hugh F. McIntyre, Dr M. Millar-Craig, Dr Dave L. Murdoch, Dr Olusola Odemuyiwa, Dr Susan Pound, Dr Roxy Senior, Dr J.D. Stephens, Dr Jonathan Swan, Dr J.T. Walsh, Dr Clive Weston
Figure 1 Trial profile.
Figure 2 Distribution of patients by (A) age and (B) left ventricular ejection fraction.
Figure 3 Time to (A) first occurrence of events (all cause death or hospital admission for a cardiovascular reason—primary endpoint) and (B) all cause death.
Figure 4 Primary outcome by subgroups (hazard ratio with 95% CI).
Baseline characteristics
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Demographics | ||
| Age (years) | 76.1 (4.8) | 76.1 (4.6) |
| Median | 75.2 | 75.3 |
| Women | 410 (38.4%) | 375 (35.3%) |
| Clinical | ||
| NYHA class I | 32 (3.0%) | 29 (2.7%) |
| II | 603 (56.5%) | 597 (56.3%) |
| III | 413 (38.7%) | 411 (38.7%) |
| IV | 19 (1.8%) | 24 (2.3%) |
| Ejection fraction (%) | 36 (13) | 36 (12) |
| Median | 33 | 34 |
| ≤35% | 683 (64.3%) | 686 (64.8%) |
| >35% | 380 (35.7%) | 372 (35.2%) |
| Haemodynamics | ||
| Heart rate (beats/min) | 79.2 (13.6) | 78.9 (13.7) |
| Sitting systolic blood pressure (mmHg) | 138.6 (20.1) | 139.5 (21.1) |
| Sitting diastolic blood pressure (mmHg) | 80.5 (10.8) | 80.6 (11.3) |
| Medical history | ||
| Smoker | 52 (4.9%) | 57 (5.4%) |
| Prior history of coronary artery disease | 735 (68.9%) | 717 (67.6%) |
| Prior myocardial infarction | 467 (43.8 %) | 463 (43.7%) |
| Prior percutaneous coronary intervention | 47 (4.4%) | 34 (3.2%) |
| Prior coronary artery bypass surgery | 101 (9.5%) | 94 (8.9%) |
| Cerebrovascular accident in the previous 3 months | 1 (0.1%) | 0 (0.0%) |
| Hypertension | 652 (61.1%) | 660 (62.3%) |
| Hyperlipidaemia | 490 (45.9%) | 484 (45.6%) |
| Atrial fibrillation | 361 (33.8%) | 377 (35.5%) |
| Diabetes | 287 (26.9%) | 268 (25.3%) |
| Medications | ||
| Diuretic | 915 (85.8%) | 907(85.5%) |
| Angiotensin converting enzyme inhibitor | 872 (81.7%) | 876 (82.6%) |
| Angiotensin II antagonist | 66 (6.2%) | 75 (7.1%) |
| Aldosterone antagonist | 307 (28.8%) | 280 (26.4%) |
| Cardiac glycoside | 415 (38.9%) | 422 (39.8%) |
| Antiarrhythmic | 122 (11.43%) | 145 (13.67%) |
| Lipid lowering drug | 217 (20.3%) | 238 (22.4%) |
| Vitamin K Antagonist | 149 (14.0%) | 164 (15.5%) |
| Aspirin | 456 (42.7%) | 441 (41.6%) |
| Calcium antagonist | 114 (10.7%) | 122 (11.5%) |
| Renal function | ||
| Creatinine (µmol/L) | 102.0 (35.1) | 103.5 (34.8) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Demographics | ||
| Age (years) | 76.1 (4.8) | 76.1 (4.6) |
| Median | 75.2 | 75.3 |
| Women | 410 (38.4%) | 375 (35.3%) |
| Clinical | ||
| NYHA class I | 32 (3.0%) | 29 (2.7%) |
| II | 603 (56.5%) | 597 (56.3%) |
| III | 413 (38.7%) | 411 (38.7%) |
| IV | 19 (1.8%) | 24 (2.3%) |
| Ejection fraction (%) | 36 (13) | 36 (12) |
| Median | 33 | 34 |
| ≤35% | 683 (64.3%) | 686 (64.8%) |
| >35% | 380 (35.7%) | 372 (35.2%) |
| Haemodynamics | ||
| Heart rate (beats/min) | 79.2 (13.6) | 78.9 (13.7) |
| Sitting systolic blood pressure (mmHg) | 138.6 (20.1) | 139.5 (21.1) |
| Sitting diastolic blood pressure (mmHg) | 80.5 (10.8) | 80.6 (11.3) |
| Medical history | ||
| Smoker | 52 (4.9%) | 57 (5.4%) |
| Prior history of coronary artery disease | 735 (68.9%) | 717 (67.6%) |
| Prior myocardial infarction | 467 (43.8 %) | 463 (43.7%) |
| Prior percutaneous coronary intervention | 47 (4.4%) | 34 (3.2%) |
| Prior coronary artery bypass surgery | 101 (9.5%) | 94 (8.9%) |
| Cerebrovascular accident in the previous 3 months | 1 (0.1%) | 0 (0.0%) |
| Hypertension | 652 (61.1%) | 660 (62.3%) |
| Hyperlipidaemia | 490 (45.9%) | 484 (45.6%) |
| Atrial fibrillation | 361 (33.8%) | 377 (35.5%) |
| Diabetes | 287 (26.9%) | 268 (25.3%) |
| Medications | ||
| Diuretic | 915 (85.8%) | 907(85.5%) |
| Angiotensin converting enzyme inhibitor | 872 (81.7%) | 876 (82.6%) |
| Angiotensin II antagonist | 66 (6.2%) | 75 (7.1%) |
| Aldosterone antagonist | 307 (28.8%) | 280 (26.4%) |
| Cardiac glycoside | 415 (38.9%) | 422 (39.8%) |
| Antiarrhythmic | 122 (11.43%) | 145 (13.67%) |
| Lipid lowering drug | 217 (20.3%) | 238 (22.4%) |
| Vitamin K Antagonist | 149 (14.0%) | 164 (15.5%) |
| Aspirin | 456 (42.7%) | 441 (41.6%) |
| Calcium antagonist | 114 (10.7%) | 122 (11.5%) |
| Renal function | ||
| Creatinine (µmol/L) | 102.0 (35.1) | 103.5 (34.8) |
Data are number of patients (%) or mean (SD). Where vital signs were missing the last observation available was used.
Baseline characteristics
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Demographics | ||
| Age (years) | 76.1 (4.8) | 76.1 (4.6) |
| Median | 75.2 | 75.3 |
| Women | 410 (38.4%) | 375 (35.3%) |
| Clinical | ||
| NYHA class I | 32 (3.0%) | 29 (2.7%) |
| II | 603 (56.5%) | 597 (56.3%) |
| III | 413 (38.7%) | 411 (38.7%) |
| IV | 19 (1.8%) | 24 (2.3%) |
| Ejection fraction (%) | 36 (13) | 36 (12) |
| Median | 33 | 34 |
| ≤35% | 683 (64.3%) | 686 (64.8%) |
| >35% | 380 (35.7%) | 372 (35.2%) |
| Haemodynamics | ||
| Heart rate (beats/min) | 79.2 (13.6) | 78.9 (13.7) |
| Sitting systolic blood pressure (mmHg) | 138.6 (20.1) | 139.5 (21.1) |
| Sitting diastolic blood pressure (mmHg) | 80.5 (10.8) | 80.6 (11.3) |
| Medical history | ||
| Smoker | 52 (4.9%) | 57 (5.4%) |
| Prior history of coronary artery disease | 735 (68.9%) | 717 (67.6%) |
| Prior myocardial infarction | 467 (43.8 %) | 463 (43.7%) |
| Prior percutaneous coronary intervention | 47 (4.4%) | 34 (3.2%) |
| Prior coronary artery bypass surgery | 101 (9.5%) | 94 (8.9%) |
| Cerebrovascular accident in the previous 3 months | 1 (0.1%) | 0 (0.0%) |
| Hypertension | 652 (61.1%) | 660 (62.3%) |
| Hyperlipidaemia | 490 (45.9%) | 484 (45.6%) |
| Atrial fibrillation | 361 (33.8%) | 377 (35.5%) |
| Diabetes | 287 (26.9%) | 268 (25.3%) |
| Medications | ||
| Diuretic | 915 (85.8%) | 907(85.5%) |
| Angiotensin converting enzyme inhibitor | 872 (81.7%) | 876 (82.6%) |
| Angiotensin II antagonist | 66 (6.2%) | 75 (7.1%) |
| Aldosterone antagonist | 307 (28.8%) | 280 (26.4%) |
| Cardiac glycoside | 415 (38.9%) | 422 (39.8%) |
| Antiarrhythmic | 122 (11.43%) | 145 (13.67%) |
| Lipid lowering drug | 217 (20.3%) | 238 (22.4%) |
| Vitamin K Antagonist | 149 (14.0%) | 164 (15.5%) |
| Aspirin | 456 (42.7%) | 441 (41.6%) |
| Calcium antagonist | 114 (10.7%) | 122 (11.5%) |
| Renal function | ||
| Creatinine (µmol/L) | 102.0 (35.1) | 103.5 (34.8) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Demographics | ||
| Age (years) | 76.1 (4.8) | 76.1 (4.6) |
| Median | 75.2 | 75.3 |
| Women | 410 (38.4%) | 375 (35.3%) |
| Clinical | ||
| NYHA class I | 32 (3.0%) | 29 (2.7%) |
| II | 603 (56.5%) | 597 (56.3%) |
| III | 413 (38.7%) | 411 (38.7%) |
| IV | 19 (1.8%) | 24 (2.3%) |
| Ejection fraction (%) | 36 (13) | 36 (12) |
| Median | 33 | 34 |
| ≤35% | 683 (64.3%) | 686 (64.8%) |
| >35% | 380 (35.7%) | 372 (35.2%) |
| Haemodynamics | ||
| Heart rate (beats/min) | 79.2 (13.6) | 78.9 (13.7) |
| Sitting systolic blood pressure (mmHg) | 138.6 (20.1) | 139.5 (21.1) |
| Sitting diastolic blood pressure (mmHg) | 80.5 (10.8) | 80.6 (11.3) |
| Medical history | ||
| Smoker | 52 (4.9%) | 57 (5.4%) |
| Prior history of coronary artery disease | 735 (68.9%) | 717 (67.6%) |
| Prior myocardial infarction | 467 (43.8 %) | 463 (43.7%) |
| Prior percutaneous coronary intervention | 47 (4.4%) | 34 (3.2%) |
| Prior coronary artery bypass surgery | 101 (9.5%) | 94 (8.9%) |
| Cerebrovascular accident in the previous 3 months | 1 (0.1%) | 0 (0.0%) |
| Hypertension | 652 (61.1%) | 660 (62.3%) |
| Hyperlipidaemia | 490 (45.9%) | 484 (45.6%) |
| Atrial fibrillation | 361 (33.8%) | 377 (35.5%) |
| Diabetes | 287 (26.9%) | 268 (25.3%) |
| Medications | ||
| Diuretic | 915 (85.8%) | 907(85.5%) |
| Angiotensin converting enzyme inhibitor | 872 (81.7%) | 876 (82.6%) |
| Angiotensin II antagonist | 66 (6.2%) | 75 (7.1%) |
| Aldosterone antagonist | 307 (28.8%) | 280 (26.4%) |
| Cardiac glycoside | 415 (38.9%) | 422 (39.8%) |
| Antiarrhythmic | 122 (11.43%) | 145 (13.67%) |
| Lipid lowering drug | 217 (20.3%) | 238 (22.4%) |
| Vitamin K Antagonist | 149 (14.0%) | 164 (15.5%) |
| Aspirin | 456 (42.7%) | 441 (41.6%) |
| Calcium antagonist | 114 (10.7%) | 122 (11.5%) |
| Renal function | ||
| Creatinine (µmol/L) | 102.0 (35.1) | 103.5 (34.8) |
Data are number of patients (%) or mean (SD). Where vital signs were missing the last observation available was used.
Compliance to treatment and reasons for discontinuation
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Patient-years of follow-up | 1863 | 1839 |
| Patient-years (%) observed/totala | 98.5% | 98.3% |
| Follow-up (months)b | 20.4 (13.7–29.1) | 19.9 (13.2–29.1) |
| Patient-years of follow-up on-drug | 1606 | 1618 |
| On-drug at end of titrationc | 1019 (95.5%) | 1026 (96.7%) |
| On-drug at 6 monthsd | 885 (82.9%) | 898 (84.6%) |
| On-drug at 12 monthse | 741 (69.5%) | 743 (70.0%) |
| On-drug at study end | 693 (65.0%) | 681 (64.2%) |
| Mean treatment dose (mg) | 7.7 (3.6) | 8.5 (3.1) |
| Maintenance dose level achieved | ||
| ≥5 mg | 815 (80.4%) | 881 (87.1%) |
| 10 mg | 688 (67.9%) | 805 (79.6%) |
| Reasons for drug discontinuationf | n=285 | n=261 |
| Intolerance to lowest dose | 24 (2.2%) | 8 (0.8%) |
| Clear contra-indication to beta blockers | 47 (4.4%) | 29 (2.7%) |
| Clear indication for beta blockers | 17 (1.6%) | 32 (3.0%) |
| Patient decision | 110 (10.3%) | 123 (11.6%) |
| Other | 83 (7.8 %) | 67 (6.3%) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Patient-years of follow-up | 1863 | 1839 |
| Patient-years (%) observed/totala | 98.5% | 98.3% |
| Follow-up (months)b | 20.4 (13.7–29.1) | 19.9 (13.2–29.1) |
| Patient-years of follow-up on-drug | 1606 | 1618 |
| On-drug at end of titrationc | 1019 (95.5%) | 1026 (96.7%) |
| On-drug at 6 monthsd | 885 (82.9%) | 898 (84.6%) |
| On-drug at 12 monthse | 741 (69.5%) | 743 (70.0%) |
| On-drug at study end | 693 (65.0%) | 681 (64.2%) |
| Mean treatment dose (mg) | 7.7 (3.6) | 8.5 (3.1) |
| Maintenance dose level achieved | ||
| ≥5 mg | 815 (80.4%) | 881 (87.1%) |
| 10 mg | 688 (67.9%) | 805 (79.6%) |
| Reasons for drug discontinuationf | n=285 | n=261 |
| Intolerance to lowest dose | 24 (2.2%) | 8 (0.8%) |
| Clear contra-indication to beta blockers | 47 (4.4%) | 29 (2.7%) |
| Clear indication for beta blockers | 17 (1.6%) | 32 (3.0%) |
| Patient decision | 110 (10.3%) | 123 (11.6%) |
| Other | 83 (7.8 %) | 67 (6.3%) |
Data are number of patients (%).
aConsidering untraced patients as followed until 15 November 2003.
bMedian (first quartile–third quartile).
cUp to 4 months after first drug intake.
dAt second maintenance visit, ∼6 months after first drug intake.
eAt fourth maintenance visit, ∼12 months after first drug intake.
fReasons other than death. Percentages of the whole ITT population. Reason not known for four and two patients in nebivolol and placebo group, respectively.
Compliance to treatment and reasons for discontinuation
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Patient-years of follow-up | 1863 | 1839 |
| Patient-years (%) observed/totala | 98.5% | 98.3% |
| Follow-up (months)b | 20.4 (13.7–29.1) | 19.9 (13.2–29.1) |
| Patient-years of follow-up on-drug | 1606 | 1618 |
| On-drug at end of titrationc | 1019 (95.5%) | 1026 (96.7%) |
| On-drug at 6 monthsd | 885 (82.9%) | 898 (84.6%) |
| On-drug at 12 monthse | 741 (69.5%) | 743 (70.0%) |
| On-drug at study end | 693 (65.0%) | 681 (64.2%) |
| Mean treatment dose (mg) | 7.7 (3.6) | 8.5 (3.1) |
| Maintenance dose level achieved | ||
| ≥5 mg | 815 (80.4%) | 881 (87.1%) |
| 10 mg | 688 (67.9%) | 805 (79.6%) |
| Reasons for drug discontinuationf | n=285 | n=261 |
| Intolerance to lowest dose | 24 (2.2%) | 8 (0.8%) |
| Clear contra-indication to beta blockers | 47 (4.4%) | 29 (2.7%) |
| Clear indication for beta blockers | 17 (1.6%) | 32 (3.0%) |
| Patient decision | 110 (10.3%) | 123 (11.6%) |
| Other | 83 (7.8 %) | 67 (6.3%) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . |
|---|---|---|
| Patient-years of follow-up | 1863 | 1839 |
| Patient-years (%) observed/totala | 98.5% | 98.3% |
| Follow-up (months)b | 20.4 (13.7–29.1) | 19.9 (13.2–29.1) |
| Patient-years of follow-up on-drug | 1606 | 1618 |
| On-drug at end of titrationc | 1019 (95.5%) | 1026 (96.7%) |
| On-drug at 6 monthsd | 885 (82.9%) | 898 (84.6%) |
| On-drug at 12 monthse | 741 (69.5%) | 743 (70.0%) |
| On-drug at study end | 693 (65.0%) | 681 (64.2%) |
| Mean treatment dose (mg) | 7.7 (3.6) | 8.5 (3.1) |
| Maintenance dose level achieved | ||
| ≥5 mg | 815 (80.4%) | 881 (87.1%) |
| 10 mg | 688 (67.9%) | 805 (79.6%) |
| Reasons for drug discontinuationf | n=285 | n=261 |
| Intolerance to lowest dose | 24 (2.2%) | 8 (0.8%) |
| Clear contra-indication to beta blockers | 47 (4.4%) | 29 (2.7%) |
| Clear indication for beta blockers | 17 (1.6%) | 32 (3.0%) |
| Patient decision | 110 (10.3%) | 123 (11.6%) |
| Other | 83 (7.8 %) | 67 (6.3%) |
Data are number of patients (%).
aConsidering untraced patients as followed until 15 November 2003.
bMedian (first quartile–third quartile).
cUp to 4 months after first drug intake.
dAt second maintenance visit, ∼6 months after first drug intake.
eAt fourth maintenance visit, ∼12 months after first drug intake.
fReasons other than death. Percentages of the whole ITT population. Reason not known for four and two patients in nebivolol and placebo group, respectively.
Primary and main secondary outcomes (time to first event)a
| . | Nebivololb (n=1067) . | Placebob (n=1061) . | HRa . | 95% CI . | P-valuea . |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All cause mortality or CV hospitalization | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.86 | 0.74–0.99 | 0.039 |
| All cause mortality contributing to primary outcomec | 76 (7.1%)/4.1 | 99 (9.3%)/5.4 | |||
| CV hospitalizations contributing to primary outcomec | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | |||
| Primary outcome, unadjusted analysis | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.85 | 0.74–0.99 | 0.034 |
| Secondary outcomes | |||||
| All cause mortality | 169 (15.8%)/9.1 | 192 (18.1%)/10.4 | 0.88 | 0.71–1.08 | 0.21 |
| CV mortality | 123 (11.5%)/6.9 | 145 (13.7%)/8.2 | 0.84 | 0.66–1.07 | 0.17 |
| Sudden cardiac deathc,d | 44 (4.1%)/2.5 | 70 (6.6%)/4.0 | |||
| Non-CV mortalityc | 26 (2.4%)/1.5 | 20 (1.9%)/1.1 | |||
| Unknown/not classifiedc,e | 20 (1.9%)/1.1 | 27 (2.5%)/1.5 | |||
| CV hospitalization | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | 0.90 | 0.76–1.06 | 0.20 |
| CV mortality or CV hospitalization | 305 (28.6%)/19.4 | 350 (33.0%)/23.2 | 0.84 | 0.72–0.98 | 0.027 |
| All cause hospitalization | 359 (33.6%)/24.4 | 364 (34.3%)/25.7 | 0.96 | 0.82–1.10 | 0.47 |
| All cause mortality or all cause hospitalization | 408 (38.2%)/26.6 | 443 (41.8%)/30.1 | 0.90 | 0.78–1.02 | 0.082 |
| . | Nebivololb (n=1067) . | Placebob (n=1061) . | HRa . | 95% CI . | P-valuea . |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All cause mortality or CV hospitalization | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.86 | 0.74–0.99 | 0.039 |
| All cause mortality contributing to primary outcomec | 76 (7.1%)/4.1 | 99 (9.3%)/5.4 | |||
| CV hospitalizations contributing to primary outcomec | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | |||
| Primary outcome, unadjusted analysis | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.85 | 0.74–0.99 | 0.034 |
| Secondary outcomes | |||||
| All cause mortality | 169 (15.8%)/9.1 | 192 (18.1%)/10.4 | 0.88 | 0.71–1.08 | 0.21 |
| CV mortality | 123 (11.5%)/6.9 | 145 (13.7%)/8.2 | 0.84 | 0.66–1.07 | 0.17 |
| Sudden cardiac deathc,d | 44 (4.1%)/2.5 | 70 (6.6%)/4.0 | |||
| Non-CV mortalityc | 26 (2.4%)/1.5 | 20 (1.9%)/1.1 | |||
| Unknown/not classifiedc,e | 20 (1.9%)/1.1 | 27 (2.5%)/1.5 | |||
| CV hospitalization | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | 0.90 | 0.76–1.06 | 0.20 |
| CV mortality or CV hospitalization | 305 (28.6%)/19.4 | 350 (33.0%)/23.2 | 0.84 | 0.72–0.98 | 0.027 |
| All cause hospitalization | 359 (33.6%)/24.4 | 364 (34.3%)/25.7 | 0.96 | 0.82–1.10 | 0.47 |
| All cause mortality or all cause hospitalization | 408 (38.2%)/26.6 | 443 (41.8%)/30.1 | 0.90 | 0.78–1.02 | 0.082 |
CV, cardiovascular.
aHR (and P-value) calculated on time to event. Analyses adjusted by gender, age, and left ventricular ejection fraction, unless otherwise specified.
bEach cell in these columns contains the number of events, the percentage of events, and the annual rate as number of events per 100 patient-years of follow-up at risk.
cNot pre-specified analyses.
dConsidered within CV mortality.
eUnknown/not classified category includes: not witnessed, not sudden cardiac death, not classified, vital status information from patients who prematurely terminated the study.
Primary and main secondary outcomes (time to first event)a
| . | Nebivololb (n=1067) . | Placebob (n=1061) . | HRa . | 95% CI . | P-valuea . |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All cause mortality or CV hospitalization | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.86 | 0.74–0.99 | 0.039 |
| All cause mortality contributing to primary outcomec | 76 (7.1%)/4.1 | 99 (9.3%)/5.4 | |||
| CV hospitalizations contributing to primary outcomec | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | |||
| Primary outcome, unadjusted analysis | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.85 | 0.74–0.99 | 0.034 |
| Secondary outcomes | |||||
| All cause mortality | 169 (15.8%)/9.1 | 192 (18.1%)/10.4 | 0.88 | 0.71–1.08 | 0.21 |
| CV mortality | 123 (11.5%)/6.9 | 145 (13.7%)/8.2 | 0.84 | 0.66–1.07 | 0.17 |
| Sudden cardiac deathc,d | 44 (4.1%)/2.5 | 70 (6.6%)/4.0 | |||
| Non-CV mortalityc | 26 (2.4%)/1.5 | 20 (1.9%)/1.1 | |||
| Unknown/not classifiedc,e | 20 (1.9%)/1.1 | 27 (2.5%)/1.5 | |||
| CV hospitalization | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | 0.90 | 0.76–1.06 | 0.20 |
| CV mortality or CV hospitalization | 305 (28.6%)/19.4 | 350 (33.0%)/23.2 | 0.84 | 0.72–0.98 | 0.027 |
| All cause hospitalization | 359 (33.6%)/24.4 | 364 (34.3%)/25.7 | 0.96 | 0.82–1.10 | 0.47 |
| All cause mortality or all cause hospitalization | 408 (38.2%)/26.6 | 443 (41.8%)/30.1 | 0.90 | 0.78–1.02 | 0.082 |
| . | Nebivololb (n=1067) . | Placebob (n=1061) . | HRa . | 95% CI . | P-valuea . |
|---|---|---|---|---|---|
| Primary outcome | |||||
| All cause mortality or CV hospitalization | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.86 | 0.74–0.99 | 0.039 |
| All cause mortality contributing to primary outcomec | 76 (7.1%)/4.1 | 99 (9.3%)/5.4 | |||
| CV hospitalizations contributing to primary outcomec | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | |||
| Primary outcome, unadjusted analysis | 332 (31.1%)/20.3 | 375 (35.3%)/23.9 | 0.85 | 0.74–0.99 | 0.034 |
| Secondary outcomes | |||||
| All cause mortality | 169 (15.8%)/9.1 | 192 (18.1%)/10.4 | 0.88 | 0.71–1.08 | 0.21 |
| CV mortality | 123 (11.5%)/6.9 | 145 (13.7%)/8.2 | 0.84 | 0.66–1.07 | 0.17 |
| Sudden cardiac deathc,d | 44 (4.1%)/2.5 | 70 (6.6%)/4.0 | |||
| Non-CV mortalityc | 26 (2.4%)/1.5 | 20 (1.9%)/1.1 | |||
| Unknown/not classifiedc,e | 20 (1.9%)/1.1 | 27 (2.5%)/1.5 | |||
| CV hospitalization | 256 (24.0%)/16.3 | 276 (26.0%)/18.3 | 0.90 | 0.76–1.06 | 0.20 |
| CV mortality or CV hospitalization | 305 (28.6%)/19.4 | 350 (33.0%)/23.2 | 0.84 | 0.72–0.98 | 0.027 |
| All cause hospitalization | 359 (33.6%)/24.4 | 364 (34.3%)/25.7 | 0.96 | 0.82–1.10 | 0.47 |
| All cause mortality or all cause hospitalization | 408 (38.2%)/26.6 | 443 (41.8%)/30.1 | 0.90 | 0.78–1.02 | 0.082 |
CV, cardiovascular.
aHR (and P-value) calculated on time to event. Analyses adjusted by gender, age, and left ventricular ejection fraction, unless otherwise specified.
bEach cell in these columns contains the number of events, the percentage of events, and the annual rate as number of events per 100 patient-years of follow-up at risk.
cNot pre-specified analyses.
dConsidered within CV mortality.
eUnknown/not classified category includes: not witnessed, not sudden cardiac death, not classified, vital status information from patients who prematurely terminated the study.
Number of patients with adverse events (first 15 adverse categories by incidence)
| . | Nebivolol (n=1067) . | Placebo (n=1061) . | Overall (n=2128) . |
|---|---|---|---|
| Cardiac failure, aggravated | 256 (24.0%) | 265 (25.0%) | 521 (24.5%) |
| Dizziness (excluding vertigo) | 166 (15.6%) | 142 (13.4%) | 308 (14.5%) |
| Hypotension | 82 (7.7%) | 76 (7.2%) | 158 (7.4%) |
| Atrial fibrillation | 78 (7.3%) | 74 (7.0%) | 152 (7.1%) |
| Dyspnoea | 70 (6.6%) | 79 (7.4%) | 149 (7.0%) |
| Bradycardia | 118 (11.1%) | 28 (2.6%) | 146 (6.9%) |
| Dyspnoea, exacerbated | 66 (6.2%) | 72 (6.8%) | 138 (6.5%) |
| Fatigue | 72 (6.7%) | 62 (5.8%) | 134 (6.3%) |
| Angina pectoris | 52 (4.9%) | 72 (6.8%) | 124 (5.8%) |
| Hypertension | 55 (5.2%) | 62 (5.8%) | 117 (5.5%) |
| Headache | 62 (5.8%) | 52 (4.9%) | 114 (5.4%) |
| Oedema lower limb | 55 (5.2%) | 24 (2.3%) | 79 (3.7%) |
| Nasopharyngitis | 43 (4.0%) | 34 (3.2%) | 77 (3.6%) |
| Unstable angina | 31 (2.9%) | 45 (4.2%) | 76 (3.6%) |
| Anaemia | 37 (3.5%) | 38 (3.6%) | 75 (3.5%) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . | Overall (n=2128) . |
|---|---|---|---|
| Cardiac failure, aggravated | 256 (24.0%) | 265 (25.0%) | 521 (24.5%) |
| Dizziness (excluding vertigo) | 166 (15.6%) | 142 (13.4%) | 308 (14.5%) |
| Hypotension | 82 (7.7%) | 76 (7.2%) | 158 (7.4%) |
| Atrial fibrillation | 78 (7.3%) | 74 (7.0%) | 152 (7.1%) |
| Dyspnoea | 70 (6.6%) | 79 (7.4%) | 149 (7.0%) |
| Bradycardia | 118 (11.1%) | 28 (2.6%) | 146 (6.9%) |
| Dyspnoea, exacerbated | 66 (6.2%) | 72 (6.8%) | 138 (6.5%) |
| Fatigue | 72 (6.7%) | 62 (5.8%) | 134 (6.3%) |
| Angina pectoris | 52 (4.9%) | 72 (6.8%) | 124 (5.8%) |
| Hypertension | 55 (5.2%) | 62 (5.8%) | 117 (5.5%) |
| Headache | 62 (5.8%) | 52 (4.9%) | 114 (5.4%) |
| Oedema lower limb | 55 (5.2%) | 24 (2.3%) | 79 (3.7%) |
| Nasopharyngitis | 43 (4.0%) | 34 (3.2%) | 77 (3.6%) |
| Unstable angina | 31 (2.9%) | 45 (4.2%) | 76 (3.6%) |
| Anaemia | 37 (3.5%) | 38 (3.6%) | 75 (3.5%) |
Number of patients with adverse events (first 15 adverse categories by incidence)
| . | Nebivolol (n=1067) . | Placebo (n=1061) . | Overall (n=2128) . |
|---|---|---|---|
| Cardiac failure, aggravated | 256 (24.0%) | 265 (25.0%) | 521 (24.5%) |
| Dizziness (excluding vertigo) | 166 (15.6%) | 142 (13.4%) | 308 (14.5%) |
| Hypotension | 82 (7.7%) | 76 (7.2%) | 158 (7.4%) |
| Atrial fibrillation | 78 (7.3%) | 74 (7.0%) | 152 (7.1%) |
| Dyspnoea | 70 (6.6%) | 79 (7.4%) | 149 (7.0%) |
| Bradycardia | 118 (11.1%) | 28 (2.6%) | 146 (6.9%) |
| Dyspnoea, exacerbated | 66 (6.2%) | 72 (6.8%) | 138 (6.5%) |
| Fatigue | 72 (6.7%) | 62 (5.8%) | 134 (6.3%) |
| Angina pectoris | 52 (4.9%) | 72 (6.8%) | 124 (5.8%) |
| Hypertension | 55 (5.2%) | 62 (5.8%) | 117 (5.5%) |
| Headache | 62 (5.8%) | 52 (4.9%) | 114 (5.4%) |
| Oedema lower limb | 55 (5.2%) | 24 (2.3%) | 79 (3.7%) |
| Nasopharyngitis | 43 (4.0%) | 34 (3.2%) | 77 (3.6%) |
| Unstable angina | 31 (2.9%) | 45 (4.2%) | 76 (3.6%) |
| Anaemia | 37 (3.5%) | 38 (3.6%) | 75 (3.5%) |
| . | Nebivolol (n=1067) . | Placebo (n=1061) . | Overall (n=2128) . |
|---|---|---|---|
| Cardiac failure, aggravated | 256 (24.0%) | 265 (25.0%) | 521 (24.5%) |
| Dizziness (excluding vertigo) | 166 (15.6%) | 142 (13.4%) | 308 (14.5%) |
| Hypotension | 82 (7.7%) | 76 (7.2%) | 158 (7.4%) |
| Atrial fibrillation | 78 (7.3%) | 74 (7.0%) | 152 (7.1%) |
| Dyspnoea | 70 (6.6%) | 79 (7.4%) | 149 (7.0%) |
| Bradycardia | 118 (11.1%) | 28 (2.6%) | 146 (6.9%) |
| Dyspnoea, exacerbated | 66 (6.2%) | 72 (6.8%) | 138 (6.5%) |
| Fatigue | 72 (6.7%) | 62 (5.8%) | 134 (6.3%) |
| Angina pectoris | 52 (4.9%) | 72 (6.8%) | 124 (5.8%) |
| Hypertension | 55 (5.2%) | 62 (5.8%) | 117 (5.5%) |
| Headache | 62 (5.8%) | 52 (4.9%) | 114 (5.4%) |
| Oedema lower limb | 55 (5.2%) | 24 (2.3%) | 79 (3.7%) |
| Nasopharyngitis | 43 (4.0%) | 34 (3.2%) | 77 (3.6%) |
| Unstable angina | 31 (2.9%) | 45 (4.2%) | 76 (3.6%) |
| Anaemia | 37 (3.5%) | 38 (3.6%) | 75 (3.5%) |
Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study.
Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure.
Cowie MR, Fox KF, Wood DA, Metcalfe C, Thompson SG, Coats AJ, Poole-Wilson PA, Sutton GC. Hospitalisation of patients with heart failure. A population-based study.
Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJ. The current cost of heart failure to the National Health Service in the UK.
Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective.
Pernenkil R, Vinson J, Shah A, Shah AS, Beckham V, Wittenberg C, Rich MW. Course and prognosis in patients ≥70 years of age with congestive heart failure and normal versus abnormal left ventricular ejection fraction.
Remme WJ, Swedberg K. Task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Comprehensive guidelines for the diagnosis and treatment of chronic heart failure.
Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C. The Task Force on Beta-Blockers of the European Society of Cardiology. Expert consensus document on beta-adrenergic receptor blockers.
Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, Eichhorn EJ. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta adrenergic blockade.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH for the U.S. Carvedilol Heart Failure Study Group. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure.
The CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS II): a randomised trial.
The MERIT-HF Study Group. Effects of metoprolol CR/XL in chronic heart failure: metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF).
Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL for the Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure.
Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure.
Ignarro LJ. Experimental evidences of nitric oxide-dependent vasodilatory activity of nebivolol, a third generation b-blocker.
Zanchetti A. Clinical pharmacodynamics of nebivolol: new evidence of nitric-oxide mediated vasodilating activity and peculiar haemodynamic properties in hypertensive patients.
Kuroedov A, Cosentino F, Luscher TF. Pharmacological mechanisms of clinically favorable properties of a selective beta1-adrenoceptor antagonist, nebivolol.
Nodari S, Metra M, Dei Cas L. Beta-blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol.
Shibata MC, Flather MD, Böhm M, Borbola J, Cohen-Solal A, Dumitrascu D, Ferrari R, Lechat P, Parkhomenko A, Soler-Soler J, Tavazzi L, Toman J, Van Veldhuisen DJ, Coats AJ, Poole-Wilson P for the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalisations in Seniors with Heart Failure (SENIORS). Rationale and design.
Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JCM. Efficacy, safety and tolerability of β-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure.
Erdmann E, Lechat P, Verkenne P, Wiemann H. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure.
Baxter AJ, Spensley A, Hildreth A, Karimova G, O’Connell JE, Gray CS. Beta blockers in older persons with heart failure: tolerability and impact on quality of life.
Rochon PA, Tu JV, Anderson GM, Gurwitz JH, Clark JP, Lau P, Szalai JP, Sykora K, Naylor CD. Rate of heart failure and 1-year survival for older people receiving low-dose-blocker therapy after myocardial infarction.
Lenzen MJ, Scholte op Reimer WJM, Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey.
Yusuf S, Pfeffer M, Sweberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left ventricular ejection fraction: the CHARM-Preserved Trial.
Wisenbaugh T, Katz I, Davis J, Essop R, Skoularigis J, Middlemost S, Rothlisberger C, Skudicky D, Sareli P. Long-term (3-month) effects of a new beta-blocker (nebivolol) on cardiac performance in dilated cardiomyopathy.
Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised, controlled trial.
The Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure.
Krum H, Ninio D, MacDonald P. Baseline predictors of tolerability to carvedilol in patients with chronic heart failure.
Mahmoudi M, McDonagh S, Poole-Wilson PA, Dubrey SW. Obstacles to the initiation of beta blockers for heart failure in a specialised clinic within a district general hospital.
Maggioni AP, Sinagra G, Opasich C, Geraci E, Gorini M, Gronda E, Lucci D, Tognoni G, Balli E, Tavazzi L for the BRING UP Investigators. Treatment of chronic heart failure with beta adrenergic blockade beyond controlled clinical trials: the BRING UP experience.
Di Lenarda A, Scherillo M, Maggioni AP, Acquarone N, Ambrosio GB, Annicchiarico M, Bellis P, Bellotti P, De Maria R, Lavecchia R, Lucci D, Mathieu G, Opasich C, Porcu M, Tavazzi L, Cafiero M. Current presentation and management of heart failure in cardiology and internal medicine hospital units: a tale of two worlds—the TEMISTOCLE study.
Author notes
Deceased.
The Institutions of the Investigators are listed in the Appendix.