-
PDF
- Split View
-
Views
-
Cite
Cite
Jorge R. Alegria, Joerg Herrmann, David R. Holmes, Amir Lerman, Charanjit S. Rihal, Myocardial bridging, European Heart Journal, Volume 26, Issue 12, June 2005, Pages 1159–1168, https://doi.org/10.1093/eurheartj/ehi203
Close - Share Icon Share
Abstract
Myocardial bridging, a congenital coronary anomaly, is a clinical condition with several possible manifestations, and its clinical relevance is debated. This article reviews current knowledge about the anatomy, pathophysiology, clinical relevance, and treatment of myocardial bridging. Myocardial bridging is present when a segment of a major epicardial coronary artery, the ‘tunnelled artery’, runs intramurally through the myocardium. With each systole, the coronary artery is compressed. Myocardial bridging has been associated with angina, arrhythmia, depressed left ventricular function, myocardial stunning, early death after cardiac transplantation, and sudden death. Evidence indicates that the intima beneath the bridge is protected from atherosclerosis, and the proximal segment is more susceptible to development of atherosclerotic lesions because of haemodynamic disturbances. New techniques (e.g. intravascular ultrasonography and intracoronary Doppler studies) have revealed new characteristics and pathophysiologic processes such as diastolic flow abnormalities. Medical treatment generally includes β-blockers. Nitrates should be avoided because symptoms may worsen. Intracoronary stents and surgery have been attempted in selected patients. Additional research is needed to define patients in whom myocardial bridging is potentially pathologic, and randomized multicentre long-term follow-up studies are needed to assess the natural history, patient selection, and therapeutic approaches.
Introduction
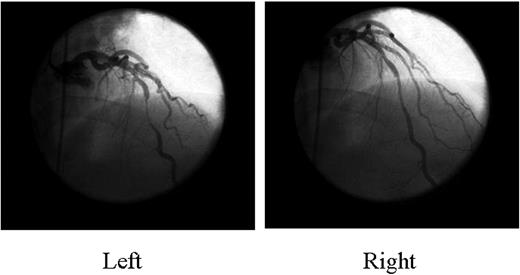
Myocardial bridging, an inborn coronary abnormality,1,2 is defined as a segment of a major epicardial coronary artery, the ‘tunnelled artery’, that goes intramurally through the myocardium beneath the muscle bridge. It was recognized at autopsy by Reyman in 17373 and first described angiographically by Portmann and Iwig in 1960.4 Myocardial bridging is generally confined to the mid left anterior descending artery (LAD),5–7 and the main angiographic finding is systolic compression of the involved epicardial coronary artery4 (Figure 1). Quantitative coronary angiography,8 intracoronary Doppler studies, and intravascular ultrasonography9–12 have documented a characteristic diastolic flow disturbance. The degree of coronary obstruction by the myocardial bridge depends on such factors as location, thickness, length of the muscle bridge, and degree of cardiac contractility. The estimated frequency that has been reported varies from 1.5 to 16% when assessed by coronary angiography, but in some autopsy series, it is as high as 80%.13,14 Traditionally, myocardial bridging has been considered a benign condition, but the following complications have been reported: ischaemia and acute coronary syndromes,12,15–29 coronary spasm,30–32 ventricular septal rupture,33 arrhythmias (including supraventricular tachycardia and ventricular tachycardia),34,35 exercise-induced atrioventricular conduction block,36 stunning,37 transient ventricular dysfunction,38,39 early death after cardiac transplantation,40 and sudden death.41–44
Our goal was to review the literature to analyse information about the anatomic aspects and pathophysiologic mechanisms of myocardial bridging, its effects on endothelial function and atherosclerosis, the clinical manifestations and implications, and the angiographic characteristics, intravascular and ultrasonographic Doppler findings, and therapeutic options.
Effect of myocardial bridges on atherosclerosis localization
Risse and Weiler45 reported that the intima of the tunnelled artery is significantly thinner (66.3 µm) than that of the proximal segment (406.6 µm) of the artery. Furthermore, changes in endothelial cell morphology indicate that the intima beneath the myocardial bridge could be protected by haemodynamic factors such as high shear stress.46 Histologic studies have indicated that the intima beneath the bridge consists only of contractile-type smooth muscle cells and an abundance of interstitial spiralled collagen. Synthetic-type smooth muscle cells are not present;47 these cells usually proliferate48 and produce collagen fibrils and elastic fibres in the intima as atherosclerosis progresses.49 The presence of contractile-type smooth muscle cells, a known physiologic phenotype, and the absence of synthetic-type smooth muscle cells in the intima of the tunnelled artery suggest a negative correlation between myocardial bridging and atherosclerosis.
The relation between alterations in the expression of vasoactive agents, including endothelial nitric oxide synthase (eNOS), endothelin-1 (ET-1), and angiotensin-converting enzyme (ACE), and the occurrence of atherosclerosis in patients with myocardial bridging was studied by Masuda et al.46 ET-1 is known to participate in the pathogenesis of atherosclerosis at all stages, even when the plaque is clinically imperceptible.50–52 ET-1 is a potent vasoconstrictor and a potent mitogen for vascular smooth muscle cells, stimulating their migration and growth. Cross-sections of the LAD with a myocardial bridge from autopsied cases were analysed from the level of the left coronary ostium to the cardiac apex and were stained with antibodies against eNOS, ET-1, and ACE. The amount of atherosclerosis present in each section was calculated using the atherosclerosis ratio (i.e. the ratio of the cross-sectional area of the intima to the cross-sectional area of the media). The extent of atherosclerosis was lower beneath the myocardial bridge than in the proximal and distal segments of the LAD, and the expression of eNOS, ET-1, and ACE was also diminished beneath the myocardial bridge. The previous data suggest that myocardial bridging is associated with the development of atherosclerosis proximal to the tunnelled artery, but currently no data demonstrate this as an independent factor.
Autopsy findings
In an unselected population, Polacek and Kralove53 found that the relative frequency of myocardial bridging exclusively involving the LAD was 70%, involving the muscular loop of the left circumflex artery 40%, and involving the right coronary artery 36%. Macroscopic observations on a total of 1056 hearts demonstrated that 23% of myocardial bridges involved the LAD and only 5.7% involved the right coronary artery.45 Autopsy studies did not demonstrate any difference in the frequency of myocardial bridges involving the LAD by age or sex.54,55
At autopsy, Ferreira et al.54 assessed the anatomy of myocardial bridges in 90 hearts from subjects (age range, stillbirth to 84 years) who did not have a history of established cardiac disease or a cardiac-related death. Myocardial bridges were identified in 50 hearts (55.6%): 35 hearts had a single bridge affecting the LAD, 10 contained two bridges, and five contained three bridges. Thirty-one were classified as ‘superficial’ (i.e. the LAD was in the interventricular groove, and before it deviated to the apex of the heart, it was crossed by the muscle bundle perpendicularly or at an acute angle). The other 10 muscle bridges were classified as ‘deep’ (i.e. the LAD deviated toward the right ventricle and was situated deeply on the interventricular septum, where it was crossed transversely, obliquely, or helically by a longitudinal muscle bundle arising from the apex of the right ventricle and inserting into the interventricular septum). The deep variant was seen less frequently and had longer muscle bundles than the superficial variant. In the deep variant, no direct contact occurred between the muscle bridge and adventitial wall of the tunnelled artery; in addition, adipose, neural, and loose connective tissues were interposed between the muscle bridge and the artery. The superficial variant may not constrict the tunnelled artery during systole; however, the deep variant, because of its relation with the LAD, could twist the artery, thus compromising its diastolic flow and producing ischaemia.
To establish, whether an intramural LAD is a simple anatomic or a pathologic variant, Morales et al.56 studied 39 hearts that had myocardial bridging but no evidence of other cardiac abnormalities. Of these hearts, 22 had gross or microscopic alterations (or both), such as interstitial fibrosis, replacement fibrosis, contraction-band necrosis, or increased vascular density, in areas of focal fibrosis in the myocardium supplied by the intramural LAD. Each of these 22 hearts had an intramural LAD placed deeply within the ventricular wall and possible attenuation of collateral blood flow because of the intramural course of the posterior descending artery, other epicardial coronary arteries, or a small right coronary artery. The myocardial changes suggested that a deep intramural LAD is abnormal and not an anatomic variant. Furthermore, the deep intramural LAD may be associated with sudden death: 13 of the 22 hearts were from victims of sudden death six of whom died suddenly during exercise.
Angiographic findings
The typical angiographic finding in myocardial bridging is systolic narrowing of an epicardial artery.57 The frequency reported in angiographic studies varies from 0.5 to 16%.58 The limited frequency of myocardial bridging observed angiographically is in contrast with that of autopsy studies, which have reported a frequency of 40–80%.59,60 The angiographic manifestation depends on the following features: the thickness and the length of the myocardial bridge; the reciprocal orientation of the coronary artery and myocardial fibres; the presence of loose connective or adipose tissue around the bridged segment; the presence of an aortic outflow tract obstruction (in which the systolic tension that develops in the myocardial bridge overcomes the intracoronary artery pressure); the intrinsic tone of the wall of the coronary artery; the presence of a proximal coronary fixed obstruction (which causes a decrease in distal intracoronary pressure); and the state of myocardial contractility.2 In uncertain cases, systolic narrowing at the myocardial bridge can be accentuated by intracoronary injection of nitroglycerin.61 With regard to the underlying mechanisms of this phenomenon, two main theories have been proposed. On the basis of the finding of an absolute reduction in systolic coronary artery dimensions at the myocardial bridge after nitroglycerin injection, Hongo et al.62 suggested that a nitroglycerin-mediated increase in vessel wall compliance and cardiac contractility leads to more extensive coronary artery compression at the myocardial bridge during systole. Herrmann et al.,63 however, were unable to confirm any change in minimal lumen diameter at the myocardial bridge in systole after nitroglycerin injection. They suggested that the wrapping of the myocardial bridge by myocardial fibres contracting in systole limits vasodilation capacity to nitroglycerin at the myocardial bridge, in marked contrast to the anatomically unrestricted conditions and vasodilation in the segments proximal and distal to it. Both theories agree with a more profound relative difference in lumen dimensions between the myocardial bridge and the adjunctive segments after nitroglycerin injection, leading to a much clearer perception of myocardial bridging.
Pathophysiologic aspects
Normally, only 15% of coronary blood flow occurs during systole, and because myocardial bridging is a systolic event on angiography, its clinical significance and relevance have been questioned. The presence of tachycardia could unmask the ischaemic effect of a myocardial bridge by shortening the diastolic period and increasing the importance of systolic blood flow.13 Also, tachycardia may worsen ischaemia because of a decrease in diastolic filling time8,23,64,65 and in coronary flow reserve (a measure of the ability to augment coronary blood flow under stress).8,66,67 According to one hypothesis, systolic kinking of the blood vessel may cause trauma to the intima and damage to the endothelium, especially at high heart rates. This, in turn, could produce platelet aggregation and vasospasm and result in an acute coronary syndrome.68–70 Pichard et al.64 described abnormal regional blood flow to the area perfused by the bridged artery with a transient decrease in flow in the great cardiac vein during rapid atrial pacing, with a simultaneous increase in total coronary sinus flow. Klues et al.10 used quantitative coronary angiography and intracoronary Doppler imaging to determine coronary flow velocities, flow reserve, and pressures in 12 patients with myocardial bridging. The haemodynamic features were characterized by phasic systolic compression with a localized peak pressure, a persistent decrease in diastolic diameter, an increase in blood flow velocities and retrograde flow, and a reduced flow reserve. A subgroup of patients underwent stent placement, which abolished the haemodynamic abnormalities and improved the ischaemic symptoms. Angiographic and intravascular ultrasonographic studies demonstrated that vessel compression during systole is followed by a delay in the increase in luminal diameter during diastole, thus affecting the predominant phase of coronary perfusion, especially during episodes of tachycardia. Indeed, Schwarz et al.8 used atrial pacing to induce tachycardia in patients who had myocardial bridging associated with at least a 70% decrease in luminal diameter of the LAD during systole. Angiographically, the maximal decrease in luminal diameter within the myocardial bridge during systole was 84% at rest, with a persistent decrease in diastolic diameter of 41%. With short-term intravenous β-blocker therapy, the decrease in diameter was less during both systole and diastole, and the angina and ischaemia improved. This effect was mediated by a reduction in both vascular compression and maximal flow velocity within the tunnelled artery. These data suggest that angina, acute coronary syndromes, and arrhythmias in patients with myocardial bridging may be explained by the reduced ischaemic threshold.
Traditionally, the area underneath the myocardial bridge has been considered to be spared from atherosclerosis and the area just proximal to it to be prone to the development of atherosclerosis along with shear stress considerations. Indeed, as outlined in a number of studies, areas of low mean shear stress and areas where blood flow departs from a laminar unidirectional pattern, including areas of oscillatory flow and flow reversal, seem to be prone to the development of atherosclerotic plaques, preceded by the development of endothelial dysfunction.71–74 Of further note, steady moderate-to-high laminar shear stress is an important survival factor for endothelial cells and the most potent stimulus for the production of nitric oxide (NO) in endothelial cells, relating to an atheroprotective effect. Alterations from this ideal blood flow and shear stress pattern lead to alterations of the expression of eNOS and ET-1 and the bioavailibility of NO and ET-1 in favour of the latter.75–77 Given the flow dynamics outlined earlier, endothelial dysfunction should be seen just proximal to the myocardial bridge, where oscillatory flow predominates; however, this is not the case in clinical practice. As shown by Preterre et al.,78 high shear stress not only stimulates antioxidant systems and NO release from cultured rat coronary endothelial cells but also increases endogenous oxidative stress. Hence, in greatly exaggerated shear stress, a detrimental increase in oxidative stress may overcome the protective effects, which may potentially be the case in myocardial bridging with high-flow, high-pressure conditions.79–81 Indeed, Kuhn et al.82 noted pronounced vasoconstriction in response to acetylcholine at the myocardial bridge rather than just proximal to it, which was subsequently confirmed by other groups. Intriguingly, an autopsy-based study found lower expressions of both eNOS and ET-1 at the myocardial bridge in comparison with the segments proximal and distal to it.46 ET-1 expression was not disproportionate to eNOS expression in this study, and by means of infusion of NG-monomethyl-L-arginine (L-NMMA), Shiode et al.83 were unable to confirm a correlation with a reduction in eNOS activity at the myocardial bridge in vivo, making an imbalance between NO and ET-1 the less likely predominant mechanism of the abnormal myocardial bridge–related vasoresponse to acetylcholine. Rather, the overall lower eNOS and ET-1 expressions at the myocardial bridge points more towards an uncoupling of the vasoresponse to acetylcholine, as outlined by Thorin84 in coronary arteries obtained from hearts with dilated and ischaemic cardiomyopathy. Reduction in the expression of muscarinic receptors or uncoupling from their intracellular signalling pathway can likewise render the endothelium unresponsive to acetylcholine, resulting in increased vasoconstriction to acetylcholine. Dissociation from the classical concept of endothelial dysfunction in myocardial bridging may relate to the dissociation from atherogenesis and prognostic implications in striking contrast to non-obstructing coronary artery disease, in which a dysfunctional response to acetylcholine indicates progression of atherosclerosis and future adverse major cardiac events. One unifying theory would be that the anatomic and haemodynamic conditions at the myocardial bridge alter vasoreactivity, yet do not allow deposition of lipid particles and hence atheroma formation and complication.
Intracoronary ultrasonography and doppler evaluation of myocardial bridges
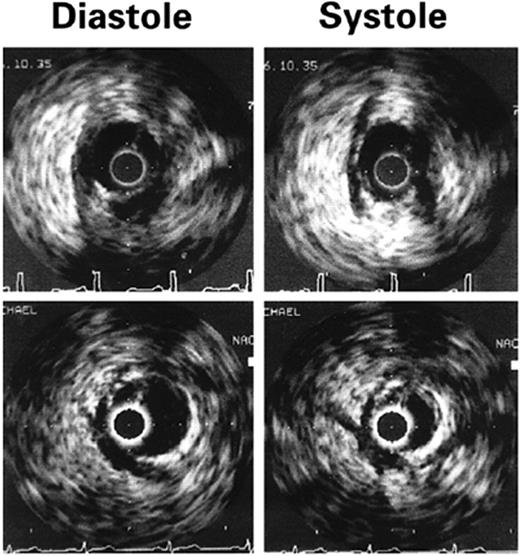
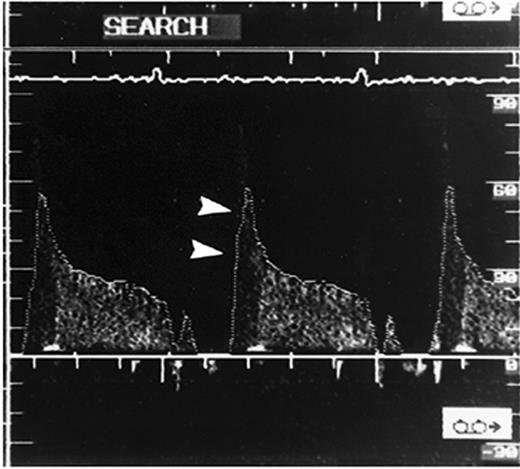
Intracoronary ultrasonography allows an accurate assessment of vascular anatomy. With Doppler studies, the coronary flow reserve measured proximal to the myocardial bridge was normal or slightly reduced [mean ratio 2.7 (normal >3.0)] and that distal to the bridge was impaired (mean ratio 2.0).85 Using intracoronary ultrasonography and pressure measurements, Ge et al.86 studied a patient who had myocardial bridging of the LAD and discovered that the pressure in the LAD segment proximal to the myocardial bridge was higher (160/26 mmHg) than that of a normal proximal segment (126/68 mmHg). The pressure distal to the myocardial bridge was 68/30 mmHg. The authors concluded that the pressure proximal to the myocardial bridge was higher than the aortic pressure, and disturbance of blood flow and high wall stress proximal to the myocardial bridge was the main contributor to the development of atherosclerosis in the proximal segment. Ge et al.67 used intravascular ultrasonography to study 62 patients with angiographically positive signs of systolic compression of a tunnelled artery and intracoronary Doppler imaging to study 48 patients with myocardial bridging. A highly specific echolucent half-moon phenomenon (Figure 2) was found over only the bridge segment that existed throughout the cardiac cycle in all 62 cases. When intracoronary Doppler imaging was used, a characteristic early diastolic ‘fingertip’ phenomenon was observed in 87% of the patients (Figure 3). The fingertip phenomenon represents the sudden reduction in myocardial tension and resistance at the level of the microcirculation, leading to an increase in blood flow volume and, with a persistent decrease in vessel diameter during early diastole, to a marked increase in blood flow velocity during this interval of the cardiac cycle. Of note, all patients had either no or reduced systolic anterograde flow, a decreased ratio of diastolic to systolic velocity, and retrograde flow in the proximal segment, which was provoked and enhanced by the injection of nitroglycerin. When the specific half-moon phenomenon was demonstrated with intravascular ultrasonography, the systolic compression could be provoked by the intracoronary administration of nitroglycerin, even if systolic compression was not revealed initially by angiography. With intravascular ultrasonography, it can be demonstrated that vessel compression within the myocardial bridge is not solely a systolic event but persists throughout a large portion of diastole.
Escaned et al.87 reported the importance of diastolic fractional flow reserve (FFR) on dobutamine challenge in a physiologic assessment of myocardial bridging. Recalling the principle that an accurate haemodynamic assessment should include inotropic stimulation,42 they compared the haemodynamic importance of myocardial bridging by using the mean and diastolic FFR with and without inotropic challenge with dobutamine. Using a conventional FFR calculated from mean pressure over the complete cardiac cycle and diastolic FFR calculated from diastolic pressures only, they compared the relevance of 12 long myocardial bridges in symptomatic patients. Diastolic FFR was compared with the mean FFR because the intracoronary systolic pressure overshoot that occurs in myocardial bridging could interfere with measurements based on mean pressures. Of the 12 patients, nine had abnormal findings on non-invasive testing at presentation, and the other three were admitted with unstable angina and electrocardiographic changes compatible with anterior ischaemia. The data suggested that (i) the angiographic and functional severities of some myocardial bridges are established only after inotropic stimulation, (ii) the development of notable diastolic pressure gradients demonstrates that myocardial bridging influences diastolic haemodynamics, and (iii) diastolic FFR identifies a proportion of haemodynamically important myocardial bridges that are not demonstrated by conventional FFR. Also, the pre-eminence of diastolic FFR over conventional FFR relies on the exclusion of systolic pressure gradients.
Clinical significance
Because myocardial bridging is a common finding at autopsy of normal subjects, it had been thought to be a benign anatomic variation. Although this malformation is present at birth, symptoms usually do not develop before the third decade; the reason for this is not clear. Bridging of the coronary arteries was observed after administration of drugs, such as nitroglycerin or a β-agonist, in up to 40% of patients with angina pectoris and normal coronary arteries.88
The diagnosis of clinically important myocardial bridging should be considered in patients who have angina and do not have the traditional risk factors and the evidence of ischaemia.89,90 However, objective signs of ischaemia cannot always be demonstrated in patients with myocardial bridging, most likely because of a large variability.2
To assess the clinical importance of an isolated myocardial bridge, Kramer et al.91 reviewed 658 normal coronary angiograms of patients with normal left ventricular function and found that 81 (12%) had a myocardial bridge of the LAD. Of these 81 patients, only 11 had a systolic reduction in luminal diameter >50% and 15 presented with typical angina. The length of the obstruction was not reported. Tests that would provide objective evidence of myocardial ischaemia were not performed. Indeed, fewer than one-third of the patients (25 of 81) had an exercise stress test, and three of the tests were positive for ischaemia. With a mean follow-up of 5 years, the survival rate was 95%, and no cardiac deaths were reported. In a study by Juilliere et al.,92 of 7467 consecutive coronary angiograms obtained over 8 years, 61 of the patients had a myocardial bridge of the LAD. Of these 61 patients, 26 had coronary artery disease, four had valvular heart disease, and three had cardiomyopathy. The long-term outcome (11 years) of 28 patients who had isolated systolic compression at baseline was studied. Patients were divided into two groups according to the per cent of systolic compression: <50% (15 patients) and ≥50% (13 patients). None of the patients had a myocardial infarction during follow-up.92 These data and others93,94 indicate that most patients with myocardial bridging have a good prognosis, but not enough long-term data are available from a large group of symptomatic patients who have a high degree of systolic and diastolic compressions and evidence of ischaemia to draw definitive conclusions.
Yano et al.95 recently evaluated the clinical significance of myocardial bridging in patients with inferior wall myocardial infarction and shock. They studied 53 patients who had single-vessel disease (right coronary artery). Each patient underwent coronary angiography for acute inferior wall myocardial infarction; reperfusion of the infarct-related artery was successful in all of them. According to multiple logistic analysis, myocardial bridging of the LAD, right ventricular myocardial infarction, and peak creatine kinase (muscle–brain component) levels were predictors of shock in acute inferior wall myocardial infarction. Coronary angiography performed after the acute event showed that myocardial bridging was not present in the same patients in whom it was observed in the acute stage. A likely explanation for this is that the compensatory increase in contractility of the anterior wall had resolved because of reperfusion of the inferior wall and the absence of hyperadrenergic status and vasoactive drugs. Because left ventricular function was not evaluated during the acute stage, the study provided no evidence that myocardial bridging was a direct cause of ischaemia. Yano et al.95 suggested that further prospective studies are needed to confirm the influence of myocardial bridging on left ventricular function in the acute phase of an inferior wall myocardial infarction presenting with shock.
Significance of myocardial bridging in hypertrophic cardiomyopathy
Myocardial bridging occurs frequently in patients with hypertrophic cardiomyopathy, with a prevalence as high as 30%.96,97 Myocardial bridging in children with hypertrophic cardiomyopathy was studied retrospectively by reviewing angiograms from 36 children, 10 of whom had myocardial bridging.20 Compared with patients without bridging, those with bridging had a greater frequency of chest pain, cardiac arrest, ventricular tachycardia, reduction in systolic pressure with exercise, greater ST-segment depression with exercise, and a greater degree of dispersion of the corrected QT interval (QTc). No association was reported between myocardial bridging and the degree of left ventricular hypertrophy or left ventricular outflow obstruction. From the time hypertrophic cardiomyopathy was diagnosed, the 5 year survival rate for children with myocardial bridging was significantly lower (P=0.004) than for children without myocardial bridging (67 and 94%, respectively). Of the total population of 36 children, nine underwent left ventricular myectomy, five received an automatic implantable cardioverter-defibrillator, and two had left and right ventricular myectomy. The tunnelled artery was surgically divided in three of the patients with myocardial bridging. In two of these three patients, thallium stress exercise testing showed reversible perfusion and a normal pattern of perfusion after unroofing of the tunnelled artery.
Mohiddin et al.98 examined the association between myocardial bridging in children with hypertrophic cardiomyopathy and myocardial perfusion abnormalities and clinical outcome. Of 57 children with hypertrophic cardiomyopathy, 23 had myocardial bridging. The mean follow-up was 8±6 years. Myocardial perfusion abnormalities were present in 14 of 30 patients (47%) who did not have myocardial bridging and in 17 of 22 patients who did have bridging. Myocardial bridging was associated with more severe septal hypertrophy, a higher ratio of the thickness of the septum to the posterior wall and a higher left ventricular outflow gradient. In 37 children (65%), the septal branches of the LAD were compressed, and this was associated with myocardial bridging, the severity of left ventricular hypertrophy, and outflow obstruction. Multivariate analysis demonstrated that thickness of the left ventricular septum and compression of the septal branches of the LAD, but not myocardial bridging, were independent predictors of abnormalities detected with thallium perfusion. Mohiddin et al.98 concluded that myocardial bridging does not produce myocardial ischaemia and may not cause arrhythmias or sudden death.
Sorajja et al.99 in a series of 425 patients with hypertrophic cardiomyopathy, evaluated 64 adults who had myocardial bridging and compared their survival with that of the 361 patients who had hypertrophic cardiomyopathy but not myocardial bridging. After a mean follow-up of 6.8 years, there was no difference between the two groups in long-term survival free of all-cause mortality and cardiac death.
Treatment
Therapeutic approaches that have been attempted for myocardial bridging include β-blockers,8 calcium channel blockers, stents,100–105 minimally invasive coronary artery bypass grafting (CABG),106 and surgical myotomy.107–109 Nitrates generally should be avoided because they increase the angiographic degree of systolic narrowing and can lead to worsening of the symptoms.62 β-blockers decrease the tachycardia and increase diastolic time, with a decrease in contractility and compression of the coronary arteries. Thus, these agents should be beneficial, although they have not been studied in randomized controlled trials.
Schwarz et al.8 studied 15 patients who had myocardial bridging in which the luminal diameter was decreased at least 70% during systole. They demonstrated that the intravenous injection of a short-acting β-blocker (esmolol) during tachypacing in symptomatic patients with severe myocardial bridging decreases Doppler flow velocities, with a return to baseline values and normalization of the diastolic-to-systolic flow velocity ratio within the bridged segment. There was also symptomatic improvement and normalization of stress-induced ST depression. Additional studies are needed to evaluate the long-term efficacy of β-blocker therapy.
Klues et al.10 demonstrated that stenting can abolish haemodynamic abnormalities and improve symptoms, although no studies demonstrate normalization of myocardial perfusion when a perfusion defect was present before stent implantation. Haager et al.103 evaluated the results of coronary stenting in 11 patients with symptomatic myocardial bridging of the central portion of the LAD. No evidence of coronary artery disease was found in the rest of the coronary arterial tree. The minimal luminal diameter increased from a mean of 0.6 (SD±0.3 mm) to 1.9 mm (SD±0.3 mm) after implantation of a stent. Intravascular ultrasonography showed a significant increase (P<0.005) in the cross-sectional area from 3.3 (SD±1.3 mm2) to 6.8 mm2 (SD±0.9 mm2) with a stent. Follow-up angiography at 7 weeks demonstrated mild-to-moderate or severe stent stenosis in ∼50% of patients; revascularization was repeated in four patients (percutaneous transluminal coronary angioplasty in two and CABG in two). The results of clinical evaluation at 2 years were normal. The frequency of in-stent restenosis requiring target vessel revascularization was 36%. In the experience of Haager et al.103 a highly flexible modified slotted tube or modular stent with good tractability was necessary. However, data comparing the mechanical properties of different stent designs are sparse. High inflation pressures may be required for optimal stent implantation, with a higher risk of coronary perforation.110,111 Intravascular ultrasonography should be used to verify optimal stent expansion.
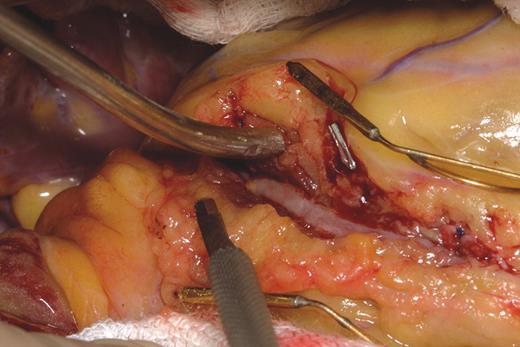
Surgical treatment with dissection of the overlying myocardium (Figure 4) should be limited to patients with symptoms that persist despite medical treatment.112 Good clinical results have been reported in a small series of patients, although serious complications such as right ventricular perforation and left ventricular aneurysm are possible.22 Intraoperative high-frequency epicardial echocardiography has been used to localize the muscle bridge and tunnelled artery.113 Patients need to be evaluated with functional studies after the procedure to assess the surgical results. Minimally invasive CABG has been reported for myocardial bridging.106 When myocardial bridging is found at the time of CABG surgery, the decision to graft beyond it is made on a case-by-case basis. If myocardial bridging is suspected of producing ischaemia, then the surgeon should proceed with dissection of the overlying myocardium.
Conclusions
Myocardial bridging is a congenital, generally benign condition that is a common angiographic and autopsy finding. The two morphologic variants are called ‘superficial’ and ‘deep’. Several case reports have associated myocardial bridging with ischaemia, arrhythmias, acute allograft failure in heart transplants, ventricular dysfunction, and sudden cardiac death. There is evidence that the arterial segment proximal to the myocardial bridge has a higher frequency of atherosclerosis, whereas the tunnelled segment is relatively spared despite evidence of endothelial dysfunction, which could predispose to coronary vasospasm and thrombosis. Intravascular ultrasonography and Doppler imaging have demonstrated that compression of the vessel within the myocardial bridge is not only a systolic event but also persist throughout diastole; it is associated with decreased coronary flow reserve. Inotropic challenge possibly should be considered for assessing the clinical importance of certain myocardial bridges. Several treatment strategies have been tried, including β-blockers, calcium channel blockers, intracoronary stents, CABG, and surgical myotomy. Additional studies are needed to assess which patients should be selected for invasive or surgical therapy.
Figure 1 Coronary angiography shows systolic compression of the mid LAD artery (myocardial bridging) (left) that almost completely resolved in diastole (right).
Figure 2 Intravascular ultrasonographic imaging of myocardial bridging during diastole and systole. A half-moon-shaped, echolucent area surrounding the bridge is seen during the entire cardiac cycle. The distance between two calibration marks is 1 mm. (From Ge et al.67 By permission of the publisher.)
Figure 3 Characteristic flow pattern in the bridge segment demonstrated by intracoronary Doppler imaging. A steep rise in the flow velocity at early diastole is followed by a sharp deceleration and subsequent plateau (‘fingertip’ phenomenon, arrowheads). No anterograde flow is observed during systole. (From Ge et al.67 By permission of the publisher.)
Figure 4 Surgical unroofing of the intramural coronary with myotomy. (Courtesy of Dr Thoralf M. Sundt III, by permission of Dr Thoralf M. Sundt III.)
References
Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance.
Angelini P, Trivellato M, Donis J, Leachman RD. Myocardial bridges: a review.
Faruqui AM, Maloy WC, Felner JM, Schlant RC, Logan WD, Symbas P. Symptomatic myocardial bridging of coronary artery.
Channer KS, Bukis E, Hartnell G, Rees JR. Myocardial bridging of the coronary arteries.
Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication.
Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Gorge G et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging.
Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Reul H, Potthast K et al. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement.
Sanchez V, Zamorano J. New approach to the diagnosis of myocardial bridging by intracoronary ultrasound and Doppler.
Kneale BJ, Stewart AJ, Coltart DJ. A case of myocardial bridging: evaluation using intracoronary ultrasound, Doppler flow measurement, and quantitative coronary angiography.
Rossi L, Dander B, Nidasio GP, Arbustini E, Paris B, Vassanelli C et al. Myocardial bridges and ischemic heart disease.
Tauth J, Sullebarger T. Myocardial infarction associated with myocardial bridging: case history and review of the literature.
Arjomand H, AlSalman J, Azain J, Amin D. Myocardial bridging of left circumflex coronary artery associated with acute myocardial infarction.
Amitani K, Yamaguchi T, Takahashi N, Uchida T, Kushikata Y, Munakata K et al. Two cases of myocardial bridge associated with myocardial ischemia [Japanese].
Schar B. Myocardial bridging: symptoms of coronary disease that sometimes is not [German].
Akdemir R, Gunduz H, Emiroglu Y, Uyan C. Myocardial bridging as a cause of acute myocardial infarction: a case report.
Yetman AT, McCrindle BW, MacDonald C, Freedom RM, Gow R. Myocardial bridging in children with hypertrophic cardiomyopathy—a risk factor for sudden death.
Yamaguchi M, Tangkawattana P, Hamlin RL. Myocardial bridging as a factor in heart disorders: critical review and hypothesis.
Iversen S, Hake U, Mayer E, Erbel R, Diefenbach C, Oelert H. Surgical treatment of myocardial bridging causing coronary artery obstruction.
Noble J, Bourassa MG, Dyrda I, Petitclerc R. Hemodynamic significance of myocardial bridging and milking effect of the anterior interventricular artery: a mild variant or source of angina? [French]
Gowda RM, Khan IA, Ansari AW, Cohen RA. Acute ST segment elevation myocardial infarction from myocardial bridging of left anterior descending coronary artery.
Bauters C, Chmait A, Tricot O, Lamblin N, Van Belle E, Lablanche JM. Images in cardiovascular medicine: coronary thrombosis and myocardial bridging.
Arnau Vives MA, Martinez Dolz LV, Almenar Bonet L, Lalaguna LA, Ten Morro F, Palencia Perez M. Myocardial bridging as a cause of acute ischemia: description of a case and review of the literature [Spanish].
Laurent G, Cottin Y, Andre F, Pichon E, Piszker G, Gerard C et al. Symptomatic myocardial bridges. Apropos of 6 cases [French].
Baldassarre S, Unger P, Renard M. Acute myocardial infarction and myocardial bridging: a case report.
Mazzu A, Di Tano G, Cogode R, Lo Presti G. Myocardial bridging involving more than one site of the left anterior descending coronary artery: an uncommon cause of acute ischemic syndrome.
Sakuma M, Kamishirado H, Inoue T, Ichihara M, Takayanagi K, Hayashi T et al. Acute myocardial infarction associated with myocardial bridge and coronary artery vasospasm.
Nayar PG, Nyamu P, Venkitachalam L, Ajit SM. Myocardial infarction due to myocardial bridging.
Berry JF, von Mering GO, Schmalfuss C, Hill JA, Kerensky RA. Systolic compression of the left anterior descending coronary artery: a case series, review of the literature, and therapeutic options including stenting.
Tio RA, Ebels T. Ventricular septal rupture caused by myocardial bridging.
Feld H, Guadanino V, Hollander G, Greengart A, Lichstein E, Shani J. Exercise-induced ventricular tachycardia in association with a myocardial bridge.
Chee TP, Jensen DP, Padnick MB, Cornell WP, Desser KB. Myocardial bridging of the left anterior descending coronary artery resulting in subendocardial infarction.
den Dulk K, Brugada P, Braat S, Heddle B, Wellens HJ. Myocardial bridging as a cause of paroxysmal atrioventricular block.
Marchionni N, Chechi T, Falai M, Margheri M, Fumagalli S. Myocardial stunning associated with a myocardial bridge.
Roul G, Sens P, Germain P, Bareiss P. Myocardial bridging as a cause of acute transient left heart dysfunction.
Galli M, Politi A, Zerboni S. “Functional myocardial bridging” and “hyperkinetic state”: a rare association as a cause of acute myocardial infarction.
Pittaluga J, de Marchena E, Posada JD, Romanelli R, Morales A. Left anterior descending coronary artery bridge: a cause of early death after cardiac transplantation.
Bestetti RB, Costa RS, Kazava DK, Oliveira JS. Can isolated myocardial bridging of the left anterior descending coronary artery be associated with sudden death during exercise?
Tio RA, Van Gelder IC, Boonstra PW, Crijns HJ. Myocardial bridging in a survivor of sudden cardiac near-death: role of intracoronary Doppler flow measurements and angiography during dobutamine stress in the clinical evaluation.
Cutler D, Wallace JM. Myocardial bridging in a young patient with sudden death.
Risse M, Weiler G. Coronary muscle bridge and its relations to local coronary sclerosis, regional myocardial ischemia and coronary spasm: a morphometric study [German].
Masuda T, Ishikawa Y, Akasaka Y, Itoh K, Kiguchi H, Ishii T. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization.
Ishii T, Asuwa N, Masuda S, Ishikawa Y, Kiguchi H, Shimada K. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: an ultrastructural study.
Campbell GR, Campbell JH. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis.
Haust MD, More RH, Movat HZ. The role of smooth muscle cells in the fibrogenesis of arteriosclerosis.
Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC Jr. Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis.
Lerman A, Hildebrand FL Jr, Aarhus LL, Burnett JC Jr. Endothelin has biological actions at pathophysiological concentrations.
Ihling C, Szombathy T, Bohrmann B, Brockhaus M, Schaefer HE, Loeffler BM. Coexpression of endothelin-converting enzyme-1 and endothelin-1 in different stages of human atherosclerosis.
Polacek P, Kralove H. Relation of myocardial bridges and loops on the coronary arteries to coronary occlusions.
Ferreira AG Jr, Trotter SE, Konig B Jr, Decourt LV, Fox K, Olsen EG. Myocardial bridges: morphological and functional aspects.
Noble J, Bourassa MG, Petitclerc R, Dyrda I. Myocardial bridging and milking effect of the left anterior descending artery: normal variant or obstruction?
Morales AR, Romanelli R, Tate LG, Boucek RJ, de Marchena E. Intramural left anterior descending coronary artery: significance of the depth of the muscular tunnel.
Amplatz K, Anderson R. Angiographic appearance of myocardial bridging of the coronary artery.
Soran O, Pamir G, Erol C, Kocakavak C, Sabah I. The incidence and significance of myocardial bridge in a prospectively defined population of patients undergoing coronary angiography for chest pain.
Kosinski A, Grzybiak M. Myocardial bridges in the human heart: morphological aspects.
Ishimori T, Raizner AE, Chahine RA, Awdeh M, Luchi RJ. Myocardial bridges in man: clinical correlations and angiographic accentuation with nitroglycerin.
Hongo Y, Tada H, Ito K, Yasumura Y, Miyatake K, Yamagishi M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: study by quantitative coronary angiography and intravascular ultrasound.
Herrmann J, Higano ST, Lennon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in vasoreactivity.
Pichard AD, Casanegra P, Marchant E, Rodriguez JA. Abnormal regional myocardial flow in myocardial bridging of the left anterior descending coronary artery.
Gallet B, Adams C, Saudemont JP, Fruchaud J, Hiltgen J. Myocardial bridge of the left anterior descending coronary artery and myocardial infarction: does coronary spasm play a part? [French.]
Frazier OH, Macris MP, Myers TJ, Duncan JM, Radovancevic B, Parnis SM et al. Improved survival after extended bridge to cardiac transplantation.
Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler.
Ciampricotti R, el Gamal M. Vasospastic coronary occlusion associated with a myocardial bridge.
Gertz SD, Uretsky G, Wajnberg RS, Navot N, Gotsman MS. Endothelial cell damage and thrombus formation after partial arterial constriction: relevance to the role of coronary artery spasm in the pathogenesis of myocardial infarction.
Maseri A, Chierchia S. Coronary artery spasm: demonstration, definition, diagnosis, and consequences.
Glagov S, Zarins C, Giddens DP, Ku DN. Hemodynamics and atherosclerosis: insights and perspectives gained from studies of human arteries.
Gibson CM, Diaz L, Kandarpa K, Sacks FM, Pasternak RC, Sandor T et al. Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries.
Sawchuk AP, Unthank JL, Davis TE, Dalsing MC. A prospective, in vivo study of the relationship between blood flow hemodynamics and atherosclerosis in a hyperlipidemic swine model.
Asakura T, Karino T. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries.
Ziegler T, Bouzourene K, Harrison VJ, Brunner HR, Hayoz D. Influence of oscillatory and unidirectional flow environments on the expression of endothelin and nitric oxide synthase in cultured endothelial cells.
Noris M, Morigi M, Donadelli R, Aiello S, Foppolo M, Todeschini M et al. Nitric oxide synthesis by cultured endothelial cells is modulated by flow conditions.
Kuchan MJ, Frangos JA. Shear stress regulates endothelin-1 release via protein kinase C and cGMP in cultured endothelial cells.
Preterre D, Morin JP, Richard V, Thuillez C. Shear stress and partial oxygen pressure independently affect NO release and redox state in cultured rat coronary endothelial cells [abstract].
Huang A, Sun D, Kaley G, Koller A. Superoxide released to high intra-arteriolar pressure reduces nitric oxide-mediated shear stress- and agonist-induced dilations.
Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase.
Ghaleh B, Hittinger L, Kim SJ, Kudej RK, Iwase M, Uechi M et al. Selective large coronary endothelial dysfunction in conscious dogs with chronic coronary pressure overload.
Kuhn FE, Reagan K, Mohler ER III, Satler LF, Lu DY, Rackley CE. Evidence for endothelial dysfunction and enhanced vasoconstriction in myocardial bridges.
Shiode N, Kato M, Teragawa H, Yamada T, Hirao H, Nomura K et al. Vasomotility and nitric oxide bioactivity of the bridging segments of the left anterior descending coronary artery.
Thorin E. Influence of nitric oxide synthase inhibition and endothelin-1 receptor blockade on acetylcholine-induced coronary artery contraction in vitro in dilated and ischemic cardiomyopathies.
Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional characteristics of myocardial bridging: a combined angiographic and intracoronary Doppler flow study.
Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements.
Escaned J, Cortes J, Flores A, Goicolea J, Alfonso F, Hernandez R et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging.
Diefenbach C, Erbel R, Treese N, Bollenbach E, Meyer J. Incidence of myocardial bridges after adrenergic stimulation and decreasing afterload in patients with angina pectoris, but normal coronary arteries [German].
Kulan K, Kulan C, Tuncer C, Komsuoglu B, Telatar M. Myocardial perfusion scintigraphy in a myocardial bridging of coronary artery.
Lee YS, Moon DH, Shin JW, Park SW, Park SJ, Lee HK. Dipyridamole TI-201 SPECT imaging in patients with myocardial bridging.
Kramer JR, Kitazume H, Proudfit WL, Sones FM Jr. Clinical significance of isolated coronary bridges: benign and frequent condition involving the left anterior descending artery.
Juilliere Y, Berder V, Suty-Selton C, Buffet P, Danchin N, Cherrier F. Isolated myocardial bridges with angiographic milking of the left anterior descending coronary artery: a long-term follow-up study.
Lozano I, Baz JA, Lopez Palop R, Pinar E, Pico F, Valdes M et al. Long-term prognosis of patients with myocardial bridge and angiographic milking of the left anterior descending coronary artery [Spanish].
Harikrishnan S, Sunder KR, Tharakan J, Titus T, Bhat A, Sivasankaran S et al. Clinical and angiographic profile and follow-up of myocardial bridges: a study of 21 cases.
Yano K, Yoshino H, Taniuchi M, Kachi E, Shimizu H, Watanuki A et al. Myocardial bridging of the left anterior descending coronary artery in acute inferior wall myocardial infarction.
Kitazume H, Kramer JR, Krauthamer D, El Tobgi S, Proudfit WL, Sones FM. Myocardial bridges in obstructive hypertrophic cardiomyopathy.
Navarro-Lopez F, Soler J, Magrina J, Esplugues E, Pare JC, Sanz G et al. Systolic compression of coronary artery in hypertrophic cardiomyopathy.
Mohiddin SA, Begley D, Shih J, Fananapazir L. Myocardial bridging does not predict sudden death in children with hypertrophic cardiomyopathy but is associated with more severe cardiac disease.
Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Tajik AJ, Holmes DR. Myocardial bridging in adult patients with hypertrophic cardiomyopathy.
Bayes A, Marti V, Auge JM. Coronary stenting for symptomatic myocardial bridging.
Zen K, Ito K, Tanabe T, Hikosaka T, Adachi Y, Kato S. Stent implantation in a case of myocardial bridging with resistant angina pectoris [Japanese].
Prendergast BD, Kerr F, Starkey IR. Normalisation of abnormal coronary fractional flow reserve associated with myocardial bridging using an intracoronary stent.
Haager PK, Schwarz ER, vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long term angiographic and clinical follow up in patients with stent implantation for symptomatic myocardial bridging.
Marti V, Ramirez J, Lamich R, Garcia J, Guiteras P, Aymat RM et al. Coronary stent placement for recurrent angina secondary to myocardial bridging [Spanish].
Stables RH, Knight CJ, McNeill JG, Sigwart U. Coronary stenting in the management of myocardial ischaemia caused by muscle bridging.
Pratt JW, Michler RE, Pala J, Brown DA. Minimally invasive coronary artery bypass grafting for myocardial muscle bridging.
Hillman ND, Mavroudis C, Backer CL, Duffy CE. Supraarterial decompression myotomy for myocardial bridging in a child.
Atmaca Y, Ozdol C, Pamir G, Kilickap M, Oral D. Successful surgical resection of a muscular bridge in a patient with nonobstructive hypertrophic cardiomyopathy–a case report.
Jeremias A, Haude M, Ge J, Gorge G, Liu F, Konorza T et al. Emergency stent implantation in the area of extensive muscle bridging of the anterior interventricular ramus after post-interventional dissection [German].
Hering D, Horstkotte D, Schwimmbeck P, Piper C, Bilger J, Schultheiss HP. Acute myocardial infarct caused by a muscle bridge of the anterior interventricular ramus: complicated course with vascular perforation after stent implantation [German].
Broderick TM, Kereiakes DJ, Whang DD, Toltzis RJ, Abbottsmith CW. Myocardial bridging may predispose to coronary perforation during rotational atherectomy.
Katznelson Y, Petchenko P, Knobel B, Cohen AJ, Kishon Y, Schachner A. Myocardial bridging: surgical technique and operative results.