-
PDF
- Split View
-
Views
-
Cite
Cite
Authors/Task Force members, Raimund Erbel, Victor Aboyans, Catherine Boileau, Eduardo Bossone, Roberto Di Bartolomeo, Holger Eggebrecht, Arturo Evangelista, Volkmar Falk, Herbert Frank, Oliver Gaemperli, Martin Grabenwöger, Axel Haverich, Bernard Iung, Athanasios John Manolis, Folkert Meijboom, Christoph A. Nienaber, Marco Roffi, Hervé Rousseau, Udo Sechtem, Per Anton Sirnes, Regula S. von Allmen, Christiaan J.M. Vrints, ESC Committee for Practice Guidelines (CPG), Jose Luis Zamorano, Stephan Achenbach, Helmut Baumgartner, Jeroen J. Bax, Héctor Bueno, Veronica Dean, Christi Deaton, Çetin Erol, Robert Fagard, Roberto Ferrari, David Hasdai, Arno Hoes, Paulus Kirchhof, Juhani Knuuti, Philippe Kolh, Patrizio Lancellotti, Ales Linhart, Petros Nihoyannopoulos, Massimo F. Piepoli, Piotr Ponikowski, Per Anton Sirnes, Juan Luis Tamargo, Michal Tendera, Adam Torbicki, William Wijns, Stephan Windecker, Document reviewers, Petros Nihoyannopoulos, Michal Tendera, Martin Czerny, John Deanfield, Carlo Di Mario, Mauro Pepi, Maria Jesus Salvador Taboada, Marc R. van Sambeek, Charalambos Vlachopoulos, Jose Luis Zamorano, Michael Grimm, Oktay Musayev, Agnès Pasquet, Zumreta Kušljugić, Maja Cikes, Georgios P. Georghiou, Josef Stasek, Henning Molgaard, Sirje Kõvask;, Ville Kytö, Guillaume Jondeau, Zviad Bakhutashvili, Yskert von Kodolitsch, Costas Tsioufis, András Temesvári, Ronen Rubinshtein, Francesco Antonini-Canterin, Olga Lunegova, Peteris Stradins, Elie Chammas, Regina Jonkaitiene, Andrew Cassar, Knut Bjørnstad, Kazimierz Widenka, Miguel Sousa Uva, Daniel Lighezan, Jovan Perunicic, Juraj Madaric, Isidre Vilacosta, Magnus Bäck, Abdallah Mahdhaoui, Recep Demirbag, Ivan Kravchenko, 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult
The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC), European Heart Journal, Volume 35, Issue 41, 1 November 2014, Pages 2873–2926, https://doi.org/10.1093/eurheartj/ehu281Close - Share Icon Share
Abbreviations and acronyms
- 3D
three-dimensional
- AAA
abdominal aortic aneurysm
- AAS
acute aortic syndrome
- ACC
American College of Cardiology
- ACE
angiotensin-converting enzyme
- AD
Aortic dissection
- ADAM
Aneurysm Detection and Management
- AHA
American Heart Association
- AJAX
Amsterdam Acute Aneurysm
- AO
aorta
- AOS
aneurysms-osteoarthritis syndrome
- ARCH
Aortic Arch Related Cerebral Hazard
- ATS
arterial tortuosity syndrome
- BAV
bicuspid aortic valve
- BSA
body surface area
- CI
confidence interval
- CoA
coarctation of the aorta
- CPG
Committee for Practice Guidelines
- CSF
cerebrospinal fluid
- CT
computed tomography
- DREAM
Dutch Randomized Aneurysm Management
- DUS
Doppler ultrasound
- EBCT
electron beam computed tomography
- ECG
electrocardiogram
- EDS
Ehlers-Danlos syndrome
- EDSIV
Ehlers-Danlos syndrome type IV
- ESC
European Society of Cardiology
- ESH
European Society of Hypertension
- EVAR
endovascular aortic repair
- FDG
18F-fluorodeoxyglucose
- FL
false lumen
- GCA
giant cell arteritis
- GERAADA
German Registry for Acute Aortic Dissection Type A
- IAD
iatrogenic aortic dissection
- IMH
intramural haematoma
- INSTEAD
Investigation of Stent Grafts in Patients with type B Aortic Dissection
- IRAD
International Registry of Aortic Dissection
- IVUS
intravascular ultrasound
- LCC
left coronary cusp
- LDS
Loeys-Dietz syndrome
- MASS
Multicentre Aneurysm Screening Study
- MESA
Multi-Ethnic Study of Atherosclerosis
- MPR
multiplanar reconstruction
- MRA
magnetic resonance angiography
- MRI
magnetic resonance imaging
- MSCT
multislice computed tomography
- NA
not applicable
- NCC
non-coronary cusp
- ns-TAAD
non-syndromic thoracic aortic aneurysms and dissection
- OR
odds ratio
- OVER
Open Versus Endovascular Repair
- OxVasc
Oxford Vascular study
- PARTNER
Placement of AoRtic TraNscathetER Valves
- PAU
penetrating aortic ulcer
- PICSS
Patent Foramen Ovale in Cryptogenic Stroke study
- PET
positron emission tomography
- RCCA
right common carotid artery
- RCC
right coronary cusp
- RCT
randomized, clinical trial
- RR
relative risk
- SIRS
systemic inflammatory response
- SMC
smooth muscle cell
- TAA
thoracic aortic aneurysm
- TAAD
thoracic aortic aneurysms and dissection
- TAI
traumatic aortic injury
- TEVAR
thoracic endovascular aortic repair
- TGF
transforming growth factor
- TI
separate thyroid artery (A. thyroidea)
- TL
true lumen
- TOE
transoesophageal echocardiography
- TS
Turner Syndrome
- TTE
transthoracic echocardiography
- UKSAT
UK Small Aneurysm Trial
- ULP
ulcer-like projection
- WARSS
Warfarin-Aspirin Recurrent Stroke Study
1. Preamble
Guidelines summarize and evaluate all available evidence at the time of the writing process, on a particular issue with the aim of assisting health professionals in selecting the best management strategies for an individual patient, with a given condition, taking into account the impact on outcome, as well as the risk-benefit-ratio of particular diagnostic or therapeutic means. Guidelines and recommendations should help the health professionals to make decisions in their daily practice. However, the final decisions concerning an individual patient must be made by the responsible health professional(s) in consultation with the patient and caregiver as appropriate.
A great number of Guidelines have been issued in recent years by the European Society of Cardiology (ESC) as well as by other societies and organisations. Because of the impact on clinical practice, quality criteria for the development of guidelines have been established in order to make all decisions transparent to the user. The recommendations for formulating and issuing ESC Guidelines can be found on the ESC website (http://www.escardio.org/guidelines-surveys/esc-guidelines/about/Pages/rules-writing.aspx). ESC Guidelines represent the official position of the ESC on a given topic and are regularly updated.
Members of this Task Force were selected by the ESC to represent professionals involved with the medical care of patients with this pathology. Selected experts in the field undertook a comprehensive review of the published evidence for management (including diagnosis, treatment, prevention and rehabilitation) of a given condition according to ESC Committee for Practice Guidelines (CPG) policy. A critical evaluation of diagnostic and therapeutic procedures was performed including assessment of the risk-benefit-ratio. Estimates of expected health outcomes for larger populations were included, where data exist. The level of evidence and the strength of recommendation of particular management options were weighed and graded according to predefined scales, as outlined in Tables 1 and 2.
The experts of the writing and reviewing panels filled in declarations of interest forms which might be perceived as real or potential sources of conflicts of interest. These forms were compiled into one file and can be found on the ESC website (http://www.escardio.org/guidelines). Any changes in declarations of interest that arise during the writing period must be notified to the ESC and updated. The Task Force received its entire financial support from the ESC without any involvement from healthcare industry.
The ESC CPG supervises and coordinates the preparation of new Guidelines produced by Task Forces, expert groups or consensus panels. The Committee is also responsible for the endorsement process of these Guidelines. The ESC Guidelines undergo extensive review by the CPG and external experts. After appropriate revisions it is approved by all the experts involved in the Task Force. The finalized document is approved by the CPG for publication in the European Heart Journal. It was developed after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The task of developing ESC Guidelines covers not only the integration of the most recent research, but also the creation of educational tools and implementation programmes for the recommendations. To implement the guidelines, condensed pocket guidelines versions, summary slides, booklets with essential messages, summary cards for non-specialists, electronic version for digital applications (smartphones etc) are produced. These versions are abridged and, thus, if needed, one should always refer to the full text version which is freely available on the ESC website. The National Societies of the ESC are encouraged to endorse, translate and implement the ESC Guidelines. Implementation programmes are needed because it has been shown that the outcome of disease may be favourably influenced by the thorough application of clinical recommendations.
Surveys and registries are needed to verify that real-life daily practice is in keeping with what is recommended in the guidelines, thus completing the loop between clinical research, writing of guidelines, disseminating them and implementing them into clinical practice.
Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies. However, the ESC Guidelines do not override in any way whatsoever the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient’s health condition and in consultation with that patient and the patient's caregiver where appropriate and/or necessary. It is also the health professional's responsibility to verify the rules and regulations applicable to drugs and devices at the time of prescription.
2. Introduction
In addition to coronary and peripheral artery diseases, aortic diseases contribute to the wide spectrum of arterial diseases: aortic aneurysms, acute aortic syndromes (AAS) including aortic dissection (AD), intramural haematoma (IMH), penetrating atherosclerotic ulcer (PAU) and traumatic aortic injury (TAI), pseudoaneurysm, aortic rupture, atherosclerotic and inflammatory affections, as well as genetic diseases (e.g. Marfan syndrome) and congenital abnormalities including the coarctation of the aorta (CoA).
Similarly to other arterial diseases, aortic diseases may be diagnosed after a long period of subclinical development or they may have an acute presentation. Acute aortic syndrome is often the first sign of the disease, which needs rapid diagnosis and decision-making to reduce the extremely poor prognosis.
Recently, the Global Burden Disease 2010 project demonstrated that the overall global death rate from aortic aneurysms and AD increased from 2.49 per 100 000 to 2.78 per 100 000 inhabitants between 1990 and 2010, with higher rates for men.1,2 On the other hand the prevalence and incidence of abdominal aortic aneurysms have declined over the last two decades. The burden increases with age, and men are more often affected than women.2
The ESC's Task Force on Aortic Dissection, published in 2001, was one of the first documents in the world relating to disease of the aorta and was endorsed by the American College of Cardiology (ACC).3 Since that time, the diagnostic methods for imaging the aorta have improved significantly, particularly by the development of multi-slice computed tomography (MSCT) and magnetic resonance imaging (MRI) technologies. Data on new endovascular and surgical approaches have increased substantially during the past 10 years. Data from multiple registries have been published, such as the International Registry of Aortic Dissection (IRAD)4 and the German Registry for Acute Aortic Dissection Type A (GERAADA),5 consensus documents,6,7 (including a recent guideline for the diagnosis and management of patients with thoracic aortic disease authored by multiple American societies),8 as well as nationwide and regional population-based studies and position papers.9–11 The ESC therefore decided to publish updated guidelines on the diagnosis and treatment of aortic diseases related to the thoracic and abdominal aorta. Emphasis is made on rapid and efficacious diagnostic strategies and therapeutic management, including the medical, endovascular, and surgical approaches, which are often combined. In addition, genetic disorders, congenital abnormalities, aortic aneurysms, and AD are discussed in more detail.
In the following section, the normal- and the ageing aorta are described. Assessment of the aorta includes clinical examination and laboratory testing, but is based mainly on imaging techniques using ultrasound, computed tomography (CT), and MRI. Endovascular therapies are playing an increasingly important role in the treatment of aortic diseases, while surgery remains necessary in many situations. In addition to acute coronary syndromes, a prompt differential diagnosis between acute coronary syndrome and AAS is difficult—but very important, because treatment of these emergency situations is very different. Thoracic- and abdominal aortic aneurysms (TAA and AAA, respectively) are often incidental findings, but screening programmes for AAA in primary care are progressively being implemented in Europe. As survival rates after an acute aortic event improve steadily, a specific section is dedicated for chronic AD and follow-up of patients after the acute phase of AAS. Special emphasis is put on genetic and congenital aortic diseases, because preventive measures play an important role in avoiding subsequent complications. Aortic diseases of elderly patients often present as thromboembolic diseases or atherosclerotic stenosis. The calcified aorta can be a major problem for surgical or interventional measures. The calcified ‘coral reef’ aorta has to be considered as an important differential diagnosis. Aortitis and aortic tumours are also discussed.
Importantly, this document highlights the value of a holistic approach, viewing the aorta as a ‘whole organ’; indeed, in many cases (e.g. genetic disorders) tandem lesions of the aorta may exist, as illustrated by the increased probability of TAA in the case of AAA, making an arbitrary distinction between the two regions—with TAAs managed in the past by ‘cardiovascular surgeons’ and AAAs by ‘vascular surgeons’—although this differentiation may exist in academic terms.
These Guidelines are the result of a close collaboration between physicians from many different areas of expertise: cardiology, radiology, cardiac and vascular surgery, and genetics. We have worked together with the aim of providing the medical community with a guide for rapid diagnosis and decision-making in aortic diseases. In the future, treatment of such patients should at best be concentrated in ‘aorta clinics’, with the involvement of a multidisciplinary team, to ensure that optimal clinical decisions are made for each individual, especially during the chronic phases of the disease. Indeed, for most aortic surgeries, a hospital volume–outcome relationship can be demonstrated. Regarding the thoracic aorta, in a prospective cardiothoracic surgery-specific clinical database including over 13 000 patients undergoing elective aortic root and aortic valve-ascending aortic procedures, an increasing institutional case volume was associated with lower unadjusted and risk-adjusted mortality.12 The operative mortality was 58% less when undergoing surgery in the highest-, rather than in the lowest-volume centre. When volume was assessed as a continuous variable, the relationship was non-linear, with a significant negative association between risk-adjusted mortality and procedural volume observed in the lower volume range (procedural volumes <30–40 cases/year).12 A hospital volume–outcome relationship analysis for acute Type A AD repair in the United States also showed a significant inverse correlation between hospital procedural volume and mortality (34% in low-volume hospitals vs. 25% in high-volume hospitals; P = 0.003) for patients undergoing urgent or emergent repair of acute Type A AD.13 A similar relationship has been reported for the thoraco-abdominal aortic aneurysm repair, demonstrating a near doubling of in-hospital mortality at low- (median volume 1 procedure/year) in comparison with high-volume hospitals (median volume 12 procedures/year; 27 vs. 15% mortality; P < 0.001)14 and intact and ruptured open descending thoracic aneurysm repair.15 Likewise, several reports have demonstrated the volume–outcome relationship for AAA interventions. In an analysis of the outcomes after AAA open repair in 131 German hospitals,16 an independent relationship between annual volume and mortality has been reported. In a nationwide analysis of outcomes in UK hospitals, elective AAA surgical repair performed in high-volume centres was significantly associated with volume-related improvements in mortality and hospital stay, while no relationship between volume and outcome was reported for ruptured AAA repairs.17 The results for endovascular therapy are more contradictory. While no volume–outcome relationship has been found for thoracic endovascular aortic repair (TEVAR),18 one report from the UK suggests such a relationship for endovascular aortic repair (EVAR).19 Overall, these data support the need to establish centres of excellence, so-called ‘aortic teams', throughout Europe; however, in emergency cases (e.g. Type A AD or ruptured AAA) the transfer of a patient should be avoided, if sufficient medical and surgical facilities and expertise are available locally.
Finally, this document lists major gaps of evidence in many situations in order to delineate key directions for further research.
3. The normal and the ageing aorta
The aorta is the ultimate conduit, carrying, in an average lifetime, almost 200 million litres of blood to the body. It is divided by the diaphragm into the thoracic and abdominal aorta (Figure 1). The aortic wall is composed histologically of three layers: a thin inner tunica intima lined by the endothelium; a thick tunica media characterized by concentric sheets of elastic and collagen fibres with the border zone of the lamina elastica interna and -externa, as well as smooth muscle cells; and the outer tunica adventitia containing mainly collagen, vasa vasorum, and lymphatics.20,21
Segments of the ascending and descending aorta. rPA = right pulmonary artery.
In addition to the conduit function, the aorta plays an important role in the control of systemic vascular resistance and heart rate, via pressure-responsive receptors located in the ascending aorta and aortic arch. An increase in aortic pressure results in a decrease in heart rate and systemic vascular resistance, whereas a decrease in aortic pressure results in an increase in heart rate and systemic vascular resistance.20
Through its elasticity, the aorta has the role of a ‘second pump’ (Windkessel function) during diastole, which is of the utmost importance—not only for coronary perfusion.
In healthy adults, aortic diameters do not usually exceed 40 mm and taper gradually downstream. They are variably influenced by several factors including age, gender, body size [height, weight, body surface area (BSA)] and blood pressure.21–26 In this regard, the rate of aortic expansion is about 0.9 mm in men and 0.7 mm in women for each decade of life.26 This slow but progressive aortic dilation over mid-to-late adulthood is thought to be a consequence of ageing, related to a higher collagen-to-elastin ratio, along with increased stiffness and pulse pressure.20,23
Current data from athletes suggest that exercise training per se has only a limited impact on physiological aortic root remodelling, as the upper limit (99th percentile) values are 40 mm in men and 34 mm in women.27
4. Assessment of the aorta
4.1 Clinical examination
While aortic diseases may be clinically silent in many cases, a broad range of symptoms may be related to different aortic diseases: The assessment of medical history should focus on an optimal understanding of the patient's complaints, personal cardiovascular risk factors, and family history of arterial diseases, especially the presence of aneurysms and any history of AD or sudden death.
Acute deep, aching or throbbing chest or abdominal pain that can spread to the back, buttocks, groin or legs, suggestive of AD or other AAS, and best described as ‘feeling of rupture’.
Cough, shortness of breath, or difficult or painful swallowing in large TAAs.
Constant or intermittent abdominal pain or discomfort, a pulsating feeling in the abdomen, or feeling of fullness after minimal food intake in large AAAs.
Stroke, transient ischaemic attack, or claudication secondary to aortic atherosclerosis.
Hoarseness due to left laryngeal nerve palsy in rapidly progressing lesions.
In some situations, physical examination can be directed by the symptoms and includes palpation and auscultation of the abdomen and flank in the search for prominent arterial pulsations or turbulent blood flow causing murmurs, although the latter is very infrequent. Blood pressure should be compared between arms, and pulses should be looked for. The symptoms and clinical examination of patients with AD will be addressed in section 6.
4.2 Laboratory testing
Baseline laboratory assessment includes cardiovascular risk factors.28 Laboratory testing plays a minor role in the diagnosis of acute aortic diseases but is useful for differential diagnoses. Measuring biomarkers early after onset of symptoms may result in earlier confirmation of the correct diagnosis by imaging techniques, leading to earlier institution of potentially life-saving management.
4.3 Imaging
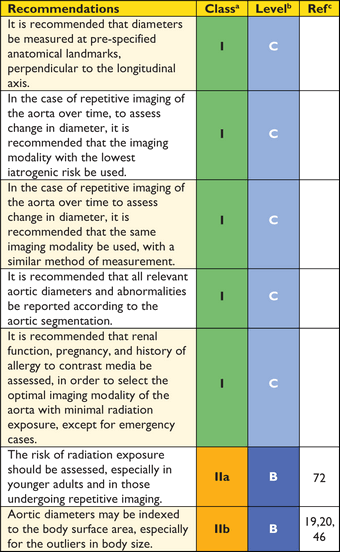
The aorta is a complex geometric structure and several measurements are useful to characterize its shape and size (Web Table 1). If feasible, diameter measurements should be made perpendicular to the axis of flow of the aorta (see Figure 2 and Web Figures 1–4).
Thoracic and abdominal aorta in a three-dimensional reconstruction (left lateral image), parasagitale multiplanar reconstruction (MPR) along the centreline (left middle part), straightened-MPR along the centreline with given landmarks (A–I) (right side), orthogonal to the centreline orientated cross-sections at the landmarks (A–J). Landmarks A–J should be used to report aortic diameters: (A) sinuses of Valsalva; (B) sinotubular junction; (C) mid ascending aorta (as indicated); (D) proximal aortic arch (aorta at the origin of the brachiocephalic trunk); (E) mid aortic arch (between left common carotid and subclavian arteries); (F) proximal descending thoracic aorta (approximately 2 cm distal to left subclavian artery); (G) mid descending aorta (level of the pulmonary arteries as easily identifiable landmarks, as indicated); (H) at diaphragm; (I) at the celiac axis origin; (J) right before aortic bifurcation. (Provided by F Nensa, Institute of Diagnostic and Interventional Radiology, Essen.)
Classification of endoleaks. Type I: Leak at graft attachment site above, below, or between graft components (Ia: proximal attachment site; Ib: distal attachment site). Type II: Aneurysm sac filling retrogradely via single (IIa) or multiple branch vessels (IIb). Type III: Leak through mechanical defect in graft, mechanical failure of the stent-graft by junctional separation of the modular components (IIIa), or fractures or holes in the endograft (IIIb). Type IV: Leak through graft fabric as a result of graft porosity. Type V: Continued expansion of aneurysm sac without demonstrable leak on imaging (endotension, controversial). (Modified from White GH, May J, Petrasek P. Semin Interv Cardiol. 2000;5:35–46107).
Classification of aortic dissection localization. Schematic drawing of aortic dissection class 1, subdivided into DeBakey Types I, II, and III.1 Also depicted are Stanford classes A and B. Type III is differentiated in subtypes III A to III C. (sub-type depends on the thoracic or abdominal involvement according to Reul et al.140)
Standardized measurements will help to better assess changes in aortic size over time and avoid erroneous findings of arterial growth. Meticulous side-by-side comparisons and measurements of serial examinations (preferably using the same imaging technique and method) are crucial, to exclude random error.
Measurements of aortic diameters are not always straightforward and some limitations inherent to all imaging techniques need to be acknowledged. First, no imaging modality has perfect resolution and the precise depiction of the aortic walls depends on whether appropriate electrocardiogram (ECG) gating is employed. Also, reliable detection of aortic diameter at the same aortic segment over time requires standardized measurement; this includes similar determination of edges (inner-to-inner, or leading edge-to-leading edge, or outer-to-outer diameter measurement, according to the imaging modality).41,43,57,58 Whether the measurement should be done during systole or diastole has not yet been accurately assessed, but diastolic images give the best reproducibility.
It is recommended that maximum aneurysm diameter be measured perpendicular to the centreline of the vessel with three-dimensional (3D) reconstructed CT scan images whenever possible (Figure 2).59 This approach offers more accurate and reproducible measurements of true aortic dimensions, compared with axial cross-section diameters, particularly in tortuous or kinked vessels where the vessel axis and the patient's cranio-caudal axis are not parallel.60 If 3D and multi-planar reconstructions are not available, the minor axis of the ellipse (smaller diameter) is generally a closer approximation of the true maximum aneurysm diameter than the major axis diameter, particularly in tortuous aneurysms.58 However, the diseased aorta is no longer necessarily a round structure, and, particularly in tortuous aneurysms, eccentricity of measurements can be caused by an oblique off-axis cut through the aorta. The minor axis measurements may underestimate the true aneurysm dimensions (Web Figures 1–4). Among patients with a minor axis of <50 mm, 7% have an aneurysmal diameter >55 mm as measured by major axis on curved multi-planar reformations.61 Compared with axial short-axis or minor-axis diameter measurements, maximum diameter measurements perpendicular to the vessel centreline have higher reproducibility.60 Inter- and intra-observer variability of CT for AAA—defined as Bland-Altman limits of agreement—are approximately 5 mm and 3 mm, respectively.43,61–63 Thus, any change of >5 mm on serial CT can be considered a significant change, but smaller changes are difficult to interpret. Compared with CT, ultrasound systematically underestimates AAA dimensions by an average of 1–3 mm.61,62,63,64,65 It is recommended that the identical imaging technique be used for serial measurements and that all serial scans be reviewed before making therapeutic decisions.
There is no consensus, for any technique, on whether the aortic wall should be included or excluded in the aortic diameter measurements, although the difference may be large, depending, for instance, on the amount of thrombotic lining of the arterial wall.65 However, recent prognostic data (especially for AAAs) are derived from measurements that include the wall.66
4.3.1 Chest X-ray
Chest X-ray obtained for other indications may detect abnormalities of aortic contour or size as an incidental finding, prompting further imaging. In patients with suspected AAS, chest X-ray may occasionally identify other causes of symptoms. Chest X-ray is, however, only of limited value for diagnosing an AAS, particularly if confined to the ascending aorta.67 In particular, a normal aortic silhouette is not sufficient to rule out the presence of an aneurysm of the ascending aorta.
4.3.2 Ultrasound
4.3.2.1 Transthoracic echocardiography
Echocardiographic evaluation of the aorta is a routine part of the standard echocardiographic examination.68 Although transthoracic echocardiography (TTE) is not the technique of choice for full assessment of the aorta, it is useful for the diagnosis and follow-up of some aortic segments. Transthoracic echocardiography is the most frequently used technique for measuring proximal aortic segments in clinical practice. The aortic root is visualized in the parasternal long-axis and modified apical five-chamber views; however, in these views the aortic walls are seen with suboptimal lateral resolution (Web Figure 1).
Modified subcostal artery may be helpful. Transthoracic echocardiography also permits assessment of the aortic valve, which is often involved in diseases of the ascending aorta. Of paramount importance for evaluation of the thoracic aorta is the suprasternal view: the aortic arch analysis should be included in all transthoracic echocardiography exams. This view primarily depicts the aortic arch and the three major supra-aortic vessels with variable lengths of the ascending and descending aorta; however, it is not possible to see the entire thoracic aorta by TTE. A short-axis view of the descending aorta can be imaged posteriorly to the left atrium in the parasternal long-axis view and in the four-chamber view. By 90° rotation of the transducer, a long-axis view is obtained and a median part of the descending thoracic aorta may be visualized. In contrast, the abdominal descending aorta is relatively easily visualized to the left of the inferior vena cava in sagittal (superior-inferior) subcostal views.
Transthoracic echocardiography is an excellent imaging modality for serial measurement of maximal aortic root diameters,57 for evaluation of aortic regurgitation, and timing for elective surgery in cases of TAA. Since the predominant area of dilation is in the proximal aorta, TTE often suffices for screening.57 Via the suprasternal view, aortic arch aneurysm, plaque calcification, thrombus, or a dissection membrane may be detectable if image quality is adequate. From this window, aortic coarctation can be suspected by continuous-wave Doppler; a patent ductus arteriosus may also be identifiable by colour Doppler. Using appropriate views (see above) aneurysmal dilation, external compression, intra-aortic thrombi, and dissection flaps can be imaged and flow patterns in the abdominal aorta assessed. The lower abdominal aorta, below the renal arteries, can be visualized to rule out AAA.
4.3.2.2 Transoesophageal echocardiography
The relative proximity of the oesophagus and the thoracic aorta permits high-resolution images with higher-frequency transoesophageal echocardiography (TOE) (Web Figure 2).68 Also, multi-plane imaging permits improved assessment of the aorta from its root to the descending aorta.68 Transoesophageal echocardiography is semi-invasive and requires sedation and strict blood pressure control, as well as exclusion of oesophageal diseases. The most important TOE views of the ascending aorta, aortic root, and aortic valve are the high TOE long-axis (at 120–150°) and short-axis (at 30–60°).68 Owing to interposition of the right bronchus and trachea, a short segment of the distal ascending aorta, just before the innominate artery, remains invisible (a ‘blind spot'). Images of the ascending aorta often contain artefacts due to reverberations from the posterior wall of the ascending aorta or the posterior wall of the right pulmonary artery, and present as aortic intraluminal horizontal lines moving in parallel with the reverberating structures, as can be ascertained by M-mode tracings.69,70 The descending aorta is easily visualized in short-axis (0°) and long-axis (90°) views from the coeliac trunk to the left subclavian artery. Further withdrawal of the probe shows the aortic arch.
Real-time 3D TOE appears to offer some advantages over two-dimensional TOE, but its clinical incremental value is not yet well-assessed.71
4.3.2.3 Abdominal ultrasound
Abdominal ultrasound (Web Figure 3) remains the mainstay imaging modality for abdominal aortic diseases because of its ability to accurately measure the aortic size, to detect wall lesions such as mural thrombus or plaques, and because of its wide availability, painlessness, and low cost. Duplex ultrasound provides additional information on aortic flow.
Colour Doppler is of great interest in the case of abdominal aorta dissection, to detect perfusion of both false and true lumen and potential re-entry sites or obstruction of tributaries (e.g. the iliac arteries).72 Nowadays Doppler tissue imaging enables the assessment of aortic compliance, and 3D ultrasound imaging may add important insights regarding its geometry, especially in the case of aneurysm. Contrast-enhanced ultrasound is useful in detecting, localizing, and quantifying endoleaks when this technique is used to follow patients after EVAR.73 For optimized imaging, abdominal aorta echography is performed after 8–12 hours of fasting that reduces intestinal gas. Usually 2.5–5 MHz curvilinear array transducers provide optimal visualization of the aorta, but the phased-array probes used for echocardiography may give sufficient image quality in many patients.74 Ultrasound evaluation of the abdominal aorta is usually performed with the patient in the supine position, but lateral decubitus positions may also be useful. Scanning the abdominal aorta usually consists of longitudinal and transverse images, from the diaphragm to the bifurcation of the aorta. Before diameter measurement, an image of the aorta should be obtained, as circular as possible, to ensure that the image chosen is perpendicular to the longitudinal axis. In this case, the anterior-posterior diameter is measured from the outer edge to the outer edge and this is considered to represent the aortic diameter. Transverse diameter measurement is less accurate. In ambiguous cases, especially if the aorta is tortuous, the anterior-posterior diameter can be measured in the longitudinal view, with the diameter perpendicular to the longitudinal axis of the aorta. In a review of the reproducibility of aorta diameter measurement,75 the inter-observer reproducibility was evaluated by the limits of agreement and ranged from ±1.9 mm to ±10.5 mm for the anterior-posterior diameter, while a variation of ±5 mm is usually considered ‘acceptable’. This should be put into perspective with data obtained during follow-up of patients, so that trivial progressions, below these limits, are clinically difficult to ascertain.
4.3.3 Computed tomography
Computed tomography plays a central role in the diagnosis, risk stratification, and management of aortic diseases. Its advantages over other imaging modalities include the short time required for image acquisition and processing, the ability to obtain a complete 3D dataset of the entire aorta, and its widespread availability (Figure 2).
Electrocardiogram (ECG)-gated acquisition protocols are crucial in reducing motion artefacts of the aortic root and thoracic aorta.76,77 High-end MSCT scanners (16 detectors or higher) are preferred for their higher spatial and temporal resolution compared with lower-end devices.8,76–79 Non-enhanced CT, followed by CT contrast-enhanced angiography, is the recommended protocol, particularly when IMH or AD are suspected. Delayed images are recommended after stent-graft repair of aortic aneurysms, to detect endoleaks. In suitable candidates scanned on 64-detector systems or higher-end devices, simultaneous CT coronary angiography may allow confirmation or exclusion of the presence of significant coronary artery disease before transcatheter or surgical repair. Computed tomography allows detection of the location of the diseased segment, the maximal diameter of dilation, the presence of atheroma, thrombus, IMH, penetrating ulcers, calcifications and, in selected cases, the extension of the disease to the aortic branches. In AD, CT can delineate the presence and extent of the dissection flap, detect areas of compromised perfusion, and contrast extravasation, indicating rupture; it can provide accurate measurements of the sinuses of Valsalva, the sinotubular junction, and the aortic valve morphology. Additionally, extending the scan field-of-view to the upper thoracic branches and the iliac and femoral arteries may assist in planning surgical or endovascular repair procedures.
In most patients with suspected AD, CT is the preferred initial imaging modality.4 In several reports, the diagnostic accuracy of CT for the detection of AD or IMH involving the thoracic aorta has been reported as excellent (pooled sensitivity 100%; pooled specificity 98%).76 Similar diagnostic accuracy has been reported for detecting traumatic aortic injury.80,81 Other features of AAS, such as penetrating ulcers, thrombus, pseudo-aneurysm, and rupture are readily depicted by CT, but data on accuracy are scarce and published reports limited.82 The drawbacks of CT angiography consist of administration of iodinated contrast agent, which may cause allergic reactions or renal failure. Also the use of ionizing radiation may limit its use in young people, especially in women, and limits its use for serial follow-up. Indeed, the average effective radiation dose during aortic computed tomography angiography (CT) is estimated to be within the 10–15 mSv range. The risk of cancer related to this radiation is substantially higher in women than in men. The risk is reduced (plateauing) beyond the age of 50 years.83
4.3.4 Positron emission tomography/computed tomography
Positron emission tomography (PET) imaging is based on the distribution of the glucose analogue 18F-fluorodeoxyglucose (FDG), which is taken up with high affinity by hypermetabolic cells (e.g. inflammatory cells), and can be used to detect vascular inflammation in large vessels. The advantages of PET may be combined with CT imaging with good resolution. Several publications suggest that FDG PET may be used to assess aortic involvement with inflammatory vascular disease (e.g. Takayasu arteritis, GCA), to detect endovascular graft infection, and to track inflammatory activity over a given period of treatment.84–86 PET may also be used as a surrogate for the activity of a lesion and as a surrogate for disease progression; however, the published literature is limited to small case series or anecdotal reports.86 The value of detection of aortic graft infection is under investigation.87
4.3.5 Magnetic resonance imaging
With its ability to delineate the intrinsic contrast between blood flow and vessel wall, MRI is well suited for diagnosing aortic diseases (Web Figure 4). The salient features necessary for clinical decision-making, such as maximal aortic diameter, shape and extent of the aorta, involvement of aortic branches in aneurysmal dilation or dissection, relationship to adjacent structures, and presence of mural thrombus, are reliably depicted by MRI.
In the acute setting, MRI is limited because it is less accessible, it is more difficult to monitor unstable patients during imaging, and it has longer acquisition times than CT.79,88 Magnetic resonance imaging does not require ionizing radiation or iodinated contrast and is therefore highly suitable for serial follow-up studies in (younger) patients with known aortic disease.
Magnetic resonance imaging of the aorta usually begins with spin-echo black blood sequences to outline its shape and diameter, and depicting an intimal flap in the presence of AD.89 Gradient-echo sequences follow in stable patients, demonstrating changes in aortic diameters during the cardiac cycle and blood flow turbulences—for instance, at entry/re-entry sites in AD, distal to bicuspid valves, or in aortic regurgitation. Contrast-enhanced MRI with intravenous gadolinium can be performed rapidly, depicting the aorta and the arch vessels as a 3D angiogram, without the need for ECG-gating. Gadolinium-enhanced sequences can be performed to differentiate slow flow from thrombus in the false lumen (FL). Importantly, the evaluation of both source and maximal intensity projection images is crucial for diagnosis because these images can occasionally fail to show the intimal flap. Evaluation of both source and maximal intensity projection images is necessary because these images may sometimes miss the dissecting membrane and the delineation of the aortic wall. Time-resolved 3D flow-sensitive MRI, with full coverage of the thoracic aorta, provides the unique opportunity to visualize and measure blood flow patterns. Quantitative parameters, such as pulse wave velocities and estimates of wall shear stress can be determined.90 The disadvantage of MRI is the difficulty of evaluating aortic valve calcification of the anchoring zones, which is important for sealing of stent grafts. The potential of gadolinium nephrotoxicity seems to be lower than for CT contrast agents, but it has to be taken into account, related to renal function.
4.3.6 Aortography
Catheter-based invasive aortography visualizes the aortic lumen, side branches, and collaterals. As a luminography technique, angiography provides exact information about the shape and size of the aorta, as well as any anomalies (Web Figures 5 and 6), although diseases of the aortic wall itself are missed, as well as thrombus-filled discrete aortic aneurysms. Additionally, angiographic techniques permit assessment and, if necessary, treatment of coronary artery and aortic branch disease. Finally, it is possible to evaluate the condition of the aortic valve and left ventricular function.
Classification of acute aortic syndrome in aortic dissection.1,141 Class 1: Classic AD with true and FL with or without communication between the two lumina. Class 2: Intramural haematoma. Class 3: Subtle or discrete AD with bulging of the aortic wall. Class 4: Ulceration of aortic plaque following plaque rupture. Class 5: Iatrogenic or traumatic AD, illustrated by a catheterinduced separation of the intima.
Flowchart for decision-making based on pre-test sensitivity of acute aortic syndrome. AAS = abdominal aortic aneurysm; AD = aortic dissection; CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
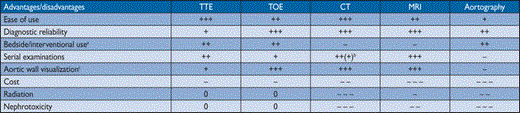
On the other hand, angiography is an invasive procedure requiring the use of contrast media. It only shows the lumen of the aorta and, hence, can miss discrete aortic aneurysms. In addition, the technique is less commonly available than TTE or CT. For this reason the non-invasive imaging modalities have largely replaced aortography in first-line diagnostic testing, both in patients with suspected AAS and with suspected or known chronic AD. However, aortography may be useful if findings by non-invasive techniques are ambiguous or incomplete. A comparison of the major imaging tools used for making the diagnosis of aortic diseases can be found in Table 3.
Comparison of methods for imaging the aorta
 |
 |
+ means a positive remark and—means a negative remark. The number of signs indicates the estimated potential value
aIVUS can be used to guide interventions (see web addenda)
b+++ only for follow-up after aortic stenting (metallic struts), otherwise limit radiation
cPET can be used to visualize suspected aortic inflammatory disease
CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
Comparison of methods for imaging the aorta
 |
 |
+ means a positive remark and—means a negative remark. The number of signs indicates the estimated potential value
aIVUS can be used to guide interventions (see web addenda)
b+++ only for follow-up after aortic stenting (metallic struts), otherwise limit radiation
cPET can be used to visualize suspected aortic inflammatory disease
CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
4.3.7 Intravascular ultrasound
To optimize visualization of the aortic wall, intravascular ultrasound (IVUS) can be used, particularly during endovascular treatment (Web Figure 7). The technique of intracardiac echocardiography is even more sophisticated (Web Figure 8).
Recommendations on imaging of the aorta
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
Recommendations on imaging of the aorta
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
4.4 Assessment of aortic stiffness
Arterial walls stiffen with age. Aortic stiffness is one of the earliest detectable manifestations of adverse structural and functional changes within the vessel wall, and is increasingly recognized as a surrogate endpoint for cardiovascular disease. Aortic stiffness has independent predictive value for all-cause and cardiovascular mortality, fatal and non-fatal coronary events, and fatal strokes in patients with various levels of cardiovascular risk, with a higher predictive value in subjects with a higher baseline cardiovascular risk.92,93 Several non-invasive methods are currently used to assess aortic stiffness, such as pulse wave velocity and augmentation index. Pulse wave velocity is calculated as the distance travelled by the pulse wave, divided by the time taken to travel the distance. Increased arterial stiffness results in increased speed of the pulse wave in the artery. Carotid-femoral pulse wave velocity is the ‘gold standard’ for measuring aortic stiffness, given its simplicity, accuracy, reproducibility, and strong predictive value for adverse outcomes. Recent hypertension guidelines have recommended measurement of arterial stiffness as part of a comprehensive evaluation of patients with hypertension, in order to detect large artery stiffening with high predictive value and reproducibility.94 Following a recent expert consensus statement in the 2013 European Society of Hypertension (ESH)/ESC Guidelines,94 a threshold for the pulse wave velocity of of >10 m/s has been suggested, which used the corrected carotid-to-femoral distance, taking into account the 20% shorter true anatomical distance travelled by the pressure wave (i.e. 0.8 × 12 m/s or 10 m/s).84 The main limitation in the interpretation of pulse wave velocity is that it is significantly influenced by blood pressure. Because elevated blood pressure increases the arterial wall tension, blood pressure becomes a confounding variable when comparing the degree of structural arterial stiffening.
5. Treatment options
5.1 Principles of medical therapy
The main aim of medical therapy in this condition is to reduce shear stress on the diseased segment of the aorta by reducing blood pressure and cardiac contractility. A large number of patients with aortic diseases have comorbidities such as coronary artery disease, chronic kidney disease, diabetes mellitus, dyslipidaemia, hypertension, etc. Therefore treatment and prevention strategies must be similar to those indicated for the above diseases. Cessation of smoking is important, as studies have shown that self-reported current smoking induced a significantly faster AAA expansion (by approximately 0.4 mm/year).95 Moderate physical activity probably prevents the progression of aortic atherosclerosis but data are sparse. To prevent blood pressure spikes, competitive sports should be avoided in patients with an enlarged aorta.
In cases of AD, treatment with intravenous beta-blocking agents is initiated to reduce the heart rate and lower the systolic blood pressure to 100–120 mm Hg, but aortic regurgitation should be excluded. Other agents may be useful in achieving the target.
In chronic conditions, blood pressure should be controlled below 140/90 mm Hg, with lifestyle changes and use of antihypertensive drugs, if necessary.94 An ideal treatment would be the one that reverses the formation of an aneurysm. In patients with Marfan syndrome, prophylactic use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitor, and angiotensin II receptor blocker seem to be able to reduce either the progression of the aortic dilation or the occurrence of complications.95–98 However, there is no evidence for the efficacy of these treatments in aortic disease of other aetiologies. Small observational studies suggest that statins may inhibit the expansion of aneurysms.99,100 Use of statins has been associated with improved survival after AAA repair, with a more than threefold reduction in the risk of cardiovascular death.101 A trial that has recently begun will show whether or not the use of statin treatment following EVAR will result in a favourable outcome.102
5.2 Endovascular therapy
5.2.1 Thoracic endovascular aortic repair
5.2.1.1 Technique
Thoracic endovascular aortic repair aims at excluding an aortic lesion (i.e. aneurysm or FL after AD) from the circulation by the implantation of a membrane-covered stent-graft across the lesion, in order to prevent further enlargement and ultimate aortic rupture.
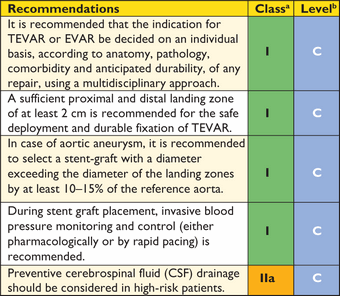
Careful pre-procedural planning is essential for a successful TEVAR procedure. Contrast-enhanced CT represents the imaging modality of choice for planning TEVAR, taking <3 mm ‘slices’ of the proximal supra-aortic branches down to the femoral arteries. The diameter (<40 mm) and length (≥20 mm) of the healthy proximal and distal landing zones are evaluated to assess the feasibility of TEVAR, along with assessment of the length of the lesion and its relationship to side branches and the iliofemoral access route.
In TAA, the stent-graft diameter should exceed the reference aortic diameter at the landing zones by at least 10–15%. In patients with Type B AD, the stent-graft is implanted across the proximal entry tear, to obstruct blood flow into the FL, depressurize the FL, and induce a process of aortic remodelling with shrinkage of the FL and enlargement of the true lumen (TL). In contrast to TAA, almost no oversizing of the stent-graft is applied.11 In situations involving important aortic side branches (e.g. left subclavian artery), TEVAR is often preceded by limited surgical revascularization of these branches (the ‘hybrid’ approach). Another option is a surgical de-branching or the use of fenestrated and branched endografts or the ‘chimney technique’. An alternative may be a single, branched stent-graft.
TEVAR is performed by retrograde transarterial advancement of a large delivery device (up to 24 F) carrying the collapsed self-expandable stent-graft. Arterial access is obtained either surgically or by the percutaneous approach, using suture-mediated access site closure. From the contralateral femoral side or from a brachial/radial access, a pigtail catheter is advanced for angiography. The stent-graft is delivered over a stiff guide wire. In AD, it may be challenging to navigate the guide wire into a narrow TL, which is essential for stent-graft placement.8 Either TOE or IVUS can be helpful in identifying the correct position of the guide wire within the TL.8 When the target position is reached, the blood pressure is reduced—either pharmacologically (nitroprusside or adenosine, <80 mm Hg systolic) or using rapid right ventricular pacing—to avoid downstream displacement, and the stent-graft is then deployed. Completion angiography is performed to detect any proximal Type I endoleak (an insufficient proximal seal), which usually mandates immediate treatment (Figure 3). More technical details are provided in the recently published joint position paper of the ESC and the European Association for Cardio-Thoracic Surgery.11
5.2.1.2 Complications
In TEVAR, vascular complications at the puncture site, as well as aortic and neurological complications, and/or endoleaks have been reported. Ideally, access site complications may be avoided by careful pre-procedural planning. Paraparesis/paraplegia and stroke rates range between 0.8–1.9% and 2.1–3.5%, respectively, and appear lower than those for open surgery.92 In order to avoid spinal cord ischaemia, vessels supplying the major spinal cord should not be covered in the elective setting (i.e. no overstenting of the left subclavian artery).103
In high-risk patients, preventive cerebrospinal fluid (CSF) drainage can be beneficial, as it has proven efficacy in spinal cord protection during open thoraco-abdominal aneurysm surgery.104 Reversal of paraplegia can be achieved by the immediate initiation of CSF drainage and pharmacological elevation of blood pressure to >90 mm Hg mean arterial pressure. Hypotensive episodes during the procedure should be avoided. Retrograde dissection of the ascending aorta after TEVAR is reported in 1.3% (0.7—2.5%) of patients.105 Endoleak describes perfusion of the excluded aortic pathology and occurs both in thoracic and abdominal (T)EVAR. Different types of endoleaks are illustrated in Figure 3. Type I and Type III endoleaks are regarded as treatment failures and warrant further treatment to prevent the continuing risk of rupture, while Type II endoleaks (Figure 3) are normally managed conservatively by a ‘wait-and-watch’ strategy to detect aneurysmal expansion, except for supra-aortic arteries.11 Endoleaks Types IV and V are indirect and have a benign course. Treatment is required in cases of aneurysm expansion.
It is important to note that plain chest radiography can be useful as an adjunct to detect material fatigue of the stent-graft and to follow ‘stent-graft’ and ‘no stent-graft'-induced changes in width, length and angulation of the thoracic aorta.
5.2.2 Abdominal endovascular aortic repair
5.2.2.1 Technique
Endovascular aortic repair is performed to prevent infrarenal AAA rupture. Similarly to TEVAR, careful pre-procedural planning by contrast-enhanced CT is essential. The proximal aortic neck (defined as the normal aortic segment between the lowest renal artery and the most cephalad extent of the aneurysm) should have a length of at least 10–15 mm and should not exceed 32 mm in diameter. Angulation above 60° of the proximal neck increases the risk of device migration and endoleak. The iliofemoral axis has to be evaluated by CT, since large delivery devices (14–24 F) are being used. Aneurysmal disease of the iliac arteries needs extension of the stent graft to the external iliac artery. Bilateral hypogastric occlusion—due to coverage of internal iliac arteries—should be avoided as it may result in buttock claudication, erectile dysfunction, and visceral ischaemia or even spinal cord ischemia.
Currently several stent-grafts are available, mostly comprising a self-expanding nitinol skeleton covered with a polyester or polytetrafluroethylene membrane. To provide an optimal seal, the stent-graft diameter should be oversized by 10–20% according to the aortic diameter at the proximal neck. Bifurcated stent-grafts are used in most cases; tube grafts may only be used in patients with localized pseudoaneurysms of the infrarenal aorta. Aorto-mono-iliac stent-grafts, with subsequent surgical femoro-femoral crossover bypass, may be time-saving in patients with acute rupture as these do not require the contralateral limb cannulation.
Choice of anaesthesia (general vs. conscious sedation) should be decided on a case-by-case basis. The stent-graft main body is introduced from the ipsilateral side, over a stiff guide wire. The contralateral access is used for a pigtail catheter for intraprocedural angiography. Fixation of the stent-graft may be either suprarenal or infrarenal, depending on the device used. After deployment of the main body, the contralateral limb is cannulated from the contralateral access or, in rare cases, from a crossover approach. The contralateral limb is introduced and implanted. After placement of all device components, stent expansion at sealing zones and connections are optimized with balloon moulding. Completion angiography is performed to check for the absence of endoleak and to confirm patency of all stent-graft components.
5.2.2.2 Complications
Immediate conversion to open surgery is required in approximately 0.6% of patients.106 Endoleak is the most common complication of EVAR. Type I and Type III endoleaks demand correction (proximal cuff or extension), while Type II endoleak may seal spontaneously in about 50% of cases. The rates of vascular injury after EVAR are low (approximately 0–3%), due to careful pre-procedural planning. The incidence of stent-graft infection after EVAR is <1%, with high mortality.
Recommendation for (thoracic) endovascular aortic repair ((T)EVAR)
 |
 |
aClass of recommendation.
bLevel of evidence.
Recommendation for (thoracic) endovascular aortic repair ((T)EVAR)
 |
 |
aClass of recommendation.
bLevel of evidence.
5.3 Surgery
5.3.1 Ascending aorta
The main principle of surgery for ascending aortic aneurysms is that of preventing the risk of dissection or rupture by restoring the normal dimension of the ascending aorta. If the aneurysm is proximally limited to the sinotubular junction and distally to the aortic arch, resection of the aneurysm and supra-commissural implantation of a tubular graft is performed under a short period of aortic clamping, with the distal anastomosis just below the aortic arch. External wrapping or reduction ascending aortoplasty (the aorta is not resected but is remodelled externally by a mesh graft) is, in general, not recommended but may be used as an alternative to reduce the aortic diameter when aortic cannulation and cardiopulmonary bypass are either not possible or not desirable. This may be the case in elderly patients with calcified aorta, in high-risk patients, or as an adjunct to other off-pump procedures.
If the aneurysm extends proximally below the sinotubular junction and one or more aortic sinuses are dilated, the surgical repair is guided by the extent of involvement of the aortic annulus and the aortic valve. In the case of a normal tricuspid aortic valve, without aortic regurgitation or central regurgitation due to annular dilation, an aortic valve-preserving technique should be performed. This includes the classic David operation with re-implantation of the aortic valve into a tubular graft or, preferably, into a graft with sinus functionality (Web Figure 9). The graft is anchored at the level of the skeletonized aortic annulus and the aortic valve is re-suspended within the graft. The procedure is completed by re-implantation of the coronary ostia. Alternatively, the classic or modified Yacoub technique may be applied, which only replaces the aortic sinus and is therefore somewhat more susceptible to late aortic annular dilation. Additional aortic annuloplasty, to reinforce the aortic annulus by using annular sutures or rings, can address this problem. In expert centres, the David technique may also be applied to patients with bicuspid aortic valve (BAV) and patients with aortic regurgitation caused by factors other than pure annular dilation. Reconstructive aortic root surgery, preserving the tricuspid valve, aims for restoration of natural haemodynamics. In patients with BAV, blood flow is altered and will remain so after repair. If there is any doubt that a durable repair can be achieved—or in the presence of aortic sclerosis or stenosis—root replacement should be performed with either a mechanical composite graft or a xenograft, according to the patient's age and potential contraindications for long-term anticoagulation.
In the case of distal aneurysmal extension to the aortic arch, leaving no neck-space for clamping the aorta at a non-diseased portion, an open distal anastomosis with the aortic arch or a hemiarch replacement should be performed. This technique allows the inspection of the aortic arch and facilitates a very distal anastomosis. A short period of antegrade cerebral perfusion and hypothermic lower body circulatory arrest are required, as the aortic arch needs to be opened and partially resected. The risk of paraplegia in aortic surgery is highly dependent on speed of repair and cross-clamp time.
Surgical mortality for isolated elective replacement of the ascending aorta (including the aortic root) ranges from 1.6–4.8% and is dependent largely on age and other well-known cardiovascular risk factors at the time of operation.108 Mortality and stroke rates for elective surgery for ascending/arch aneurysms are in the range of 2.4–3.0%.109 For patients under 55 years of age, mortality and stroke rates are as low as 1.2% and 0.6–1.2%, respectively.110
5.3.2 Aortic arch
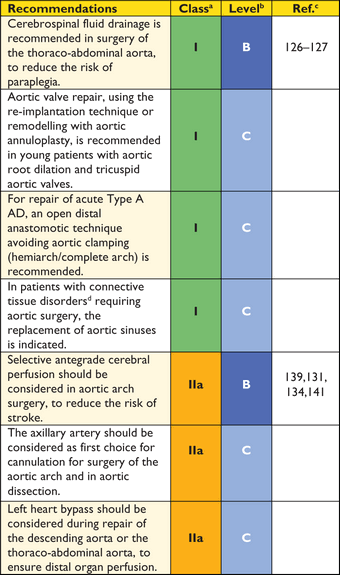
Several procedures and techniques have significantly lowered the inherent risk of aortic arch surgery, both for aneurysms and ADs. Importantly, the continuous use of antegrade cerebral perfusion,98–101 including the assessment of transcranial oxygen saturation,102 has proven itself as safe cerebral protection, even in prolonged periods (>60 min) of circulatory arrest. The axillary artery should be considered as first choice for cannulation for surgery of the aortic arch and in AD. Innovative arch prostheses, including branching for supra-aortic vessel reconnection,108 have made the timing of arch reconstruction more predictable, allowing moderate (26–28°C) rather than deep (20–22°C) hypothermia under extracorporeal circulation.111,112 This is the case for the majority of reconstructions, including acute and chronic AD, requiring total arch replacement and arrest times from 40–60 minutes. The precautions for this procedure resemble those formerly applied for partial arch repair, requiring much shorter periods of circulatory arrest (<20 minutes). Various extents and variants of aortic rerouting (left subclavian, left common carotid and finally brachiocephalic trunk, autologous vs. alloplastic) might also be used. Nowadays, many arch replacements are re-operations for dilated aneurysms after Type A AD following limited ascending aorta replacement or proximal arch repair performed in emergency.
Extensive repair including graft replacement of the ascending aorta and aortic arch and integrated stent grafting of the descending aorta108 (‘frozen elephant trunk') was introduced as a single-stage procedure.103,105 The ‘frozen elephant trunk’ is increasingly applied for this disease entity if complete ascending-, arch-, and descending AD are diagnosed in otherwise uncomplicated patients.113–117 Originally designed for repair of chronic aneurysm, the hybrid approach, consisting of a single graft, is also applied, more often now in the setting of acute dissection (Web Figures 10 and 11).118–121
5.3.3 Descending aorta
The surgical approach to the descending aorta is a left thoracotomy between the fourth and seventh intercostal spaces, depending on the extension of the aortic pathology (Web Figure 12). Established methods for operation of the descending aorta include the left heart bypass technique, the partial bypass, and the operation in deep hypothermic circulatory arrest. The simple ‘clamp and sew’ technique may not be advisable because the risk of post-operative neurological deficit, mesenteric and renal ischaemia is significant when the aortic cross-clamp procedure exceeds 30 minutes.122,123 In contrast, the left heart bypass technique provides distal aortic perfusion (by means of a centrifugal pump) during aortic clamping, which drains through cannulation of the left atrial appendage or preferably the left pulmonary veins and returns blood through cannulation of the distal aorta or femoral artery. A similar technique is the partial bypass technique, where cardiopulmonary bypass is initiated via cannulation of the femoral artery and vein and ensures perfusion and oxygenation of the organs distal to the aortic clamp. In contrast to the left heart bypass technique, this method requires full heparinization due to the cardiopulmonary bypass system used.124
The technique of deep hypothermic circulatory arrest has to be used when clamping of the descending aorta distal to the left subclavian artery—or between the carotid artery and the left subclavian artery—is not feasible because the aortic lesion includes the aortic arch. At a core temperature of 18°C the proximal anastomosis is performed; thereafter the Dacron prosthesis is clamped and the supra-aortic branches are perfused via a side-graft with 2.5 L/min. After accomplishment of the distal anastomosis, the clamp is removed from the prosthesis and complete perfusion and re-warming are started.124
5.3.4 Thoraco-abdominal aorta
When the disease affects both the descending thoracic and abdominal aorta, the surgical approach is a left thoracotomy extended to paramedian laparotomy. This access ensures exposure of the whole aorta, from the left subclavian artery to the iliac arteries (Web Figures 12 and 13). When the aortic disease starts distal to the aortic arch and clamping is feasible, the left heart bypass technique is a proven method that can be performed in experienced centres with excellent results.125–128 The advantage of this method is that it maintains distal aortic perfusion during aortic cross-clamping, including selective perfusion of mesenteric visceral and renal arteries.129–131 Owing to the protective effect of hypothermia, other adjunctive methods are unnecessary.
The risk of paraplegia after thoraco-abdominal repair is in the range of 6–8%,131,132 and procedural as well as systemic measures are beneficial in preventing this disastrous complication.133,134 These measures include permissive systemic hypothermia (34°C), re-attachment of distal intercostal arteries between T8 and L1, and the pre-operative placement of cerebrospinal fluid drainage. Drainage reduces the rate of paraplegia in patients with thoraco-abdominal aneuryms and its continuation up to 72 hours post-operatively is recommended, to prevent delayed onset of paraplegia.135–138
5.3.5 Abdominal aorta
Open abdominal aortic repair usually involves a standard median laparotomy, but may also be performed through a left retroperitoneal approach. The aorta is dissected, in particular at the aortic neck and the distal anastomotic sites. After heparinization, the aorta is cross-clamped above, below, or in between the renal arteries, depending on the proximal extent of the aneurysm. Renal ischaemia should not exceed 30 minutes, otherwise preventive measures should be taken (i.e. cold renal perfusion). The aneurysmal aorta is replaced either by a tube or bifurcated graft, according to the extent of aneurysmal disease into the iliac arteries. If the common iliac arteries are involved, the graft is anastomosed to the external iliac arteries and revascularization of the internal iliac arteries provided via separate bypass grafts.
Colonic ischaemia is a potential problem in the repair of AAA. A patent inferior mesenteric artery with pulsatile back-bleeding suggests a competent mesenteric collateral circulation and, consequently, the inferior mesenteric artery may be ligated; however, if the artery is patent and only poor back-bleeding present, re-implantation into the aortic graft must be considered, to prevent left colonic ischaemia. A re-implantation of the inferior mesenteric artery may also be necessary if one internal iliac artery has to be ligated.
The excluded aneurysm is not resected, but is closed over the graft, which has a haemostatic effect and ensures that the duodenum is not in contact with the graft, as this may lead to erosion and a possible subsequent aorto-enteric fistula.
Recommendations for surgical techniques in aortic disease
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dEhlers-Danlos IV -, Marfan- or Loeys-Dietz syndromes.
Recommendations for surgical techniques in aortic disease
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dEhlers-Danlos IV -, Marfan- or Loeys-Dietz syndromes.
6. Acute thoracic aortic syndromes
6.1 Definition
Acute aortic syndromes are defined as emergency conditions with similar clinical characteristics involving the aorta. There is a common pathway for the various manifestations of AAS that eventually leads to a breakdown of the intima and media. This may result in IMH, PAU, or in separation of aortic wall layers, leading to AD or even thoracic aortic rupture.3 Ruptured AAA is also part of the full picture of AAS, but it is presented in section 7.2 because of its specific presentation and management.
6.2 Pathology and classification
Acute aortic syndromes occur when either a tear or an ulcer allows blood to penetrate from the aortic lumen into the media or when a rupture of vasa vasorum causes a bleed within the media. The inflammatory response to blood in the media may lead to aortic dilation and rupture. Figure 4 displays the Stanford and the DeBakey classifications.140 The most common features of AAS are displayed in Figure 5.141 Acute AD (<14 days) is distinct from sub-acute (15–90 days), and chronic aortic dissection (>90 days) (see section 12).
6.3 Acute aortic dissection
6.3.1 Definition and classification
Aortic dissection is defined as disruption of the medial layer provoked by intramural bleeding, resulting in separation of the aortic wall layers and subsequent formation of a TL and an FL with or without communication. In most cases, an intimal tear is the initiating condition, resulting in tracking of the blood in a dissection plane within the media. This process is followed either by an aortic rupture in the case of adventitial disruption or by a re-entering into the aortic lumen through a second intimal tear. The dissection can be either antegrade or retrograde. The present Guidelines will apply the Stanford classification unless stated otherwise. This classification takes into account the extent of dissection, rather than the location of the entry tear. The propagation can also affect side branches. Other complications include tamponade, aortic valve regurgitation, and proximal or distal malperfusion syndromes.4,142–144 The inflammatory response to thrombus in the media is likely to initiate further necrosis and apoptosis of smooth muscle cells and degeneration of elastic tissue, which potentiates the risk of medial rupture.
6.3.2 Epidemiology
Up-to-date data on the epidemiology of AD are scarce. In the Oxford Vascular study, the incidence of AD is estimated at six per hundred thousand persons per year.10 This incidence is higher in men than in women and increases with age.9 The prognosis is poorer in women, as a result of atypical presentation and delayed diagnosis. The most common risk factor associated with AD is hypertension, observed in 65–75% of individuals, mostly poorly controlled.4,142–145 In the IRAD registry, the mean age was 63 years; 65% were men. Other risk factors include pre-existing aortic diseases or aortic valve disease, family history of aortic diseases, history of cardiac surgery, cigarette smoking, direct blunt chest trauma and use of intravenous drugs (e.g. cocaine and amphetamines). An autopsy study of road accident fatalities found that approximately 20% of victims had a ruptured aorta.146
6.3.3 Clinical presentation and complications
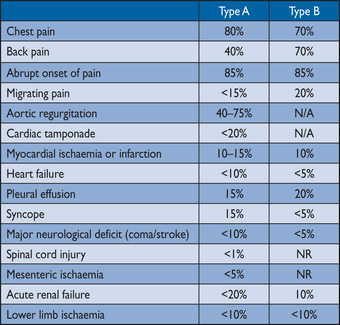
Chest pain
is the most frequent symptom of acute AD. Abrupt onset of severe chest and/or back pain is the most typical feature. The pain may be sharp, ripping, tearing, knife-like, and typically different from other causes of chest pain; the abruptness of its onset is the most specific characteristic (Table 4).4,146 The most common site of pain is the chest (80%), while back and abdominal pain are experienced in 40% and 25% of patients, respectively. Anterior chest pain is more commonly associated with Type A AD, whereas patients with Type B dissection present more frequently with pain in the back or abdomen.147,148 The clinical presentations of the two types of AD may frequently overlap. The pain may migrate from its point of origin to other sites, following the dissection path as it extends through the aorta. In IRAD, migrating pain was observed in <15% of patients with acute Type A AD, and in approximately 20% of those with acute Type B.
Main clinical presentations and complications of patients with acute aortic dissection
 |
 |
NR = not reported; NA = not applicable. Percentages are approximated.
Main clinical presentations and complications of patients with acute aortic dissection
 |
 |
NR = not reported; NA = not applicable. Percentages are approximated.
Although any pulse deficit may be as frequent as 30% in patients with Type A AD and 15% in those with Type B, overt lower limb ischaemia is rare.
Multiple reports have described signs and symptoms of end-organ dysfunction related to AD. Patients with acute Type A AD suffer double the mortality of individuals presenting with Type B AD (25% and 12%, respectively).146 Cardiac complications are the most frequent in patients with AD. Aortic regurgitation may accompany 40–75% of cases with Type A AD.148–150 After acute aortic rupture, aortic regurgitation is the second most common cause of death in patients with AD. Patients with acute severe aortic regurgitation commonly present with heart failure and cardiogenic shock.
Aortic regurgitation
in AD includes dilation of the aortic root and annulus, tearing of the annulus or valve cusps, downward displacement of one cusp below the line of the valve closure, loss of support of the cusp, and physical interference in the closure of the aortic valve by an intimal flap. Pericardial tamponade may be observed in <20% of patients with acute Type A AD. This complication is associated with a doubling of mortality.144,145
Myocardial ischaemia
or infarction may be present in 10–15% of patients with AD and may result from aortic FL expansion, with subsequent compression or obliteration of coronary ostia or the propagation of the dissection process into the coronary tree.151 In the presence of a complete coronary obstruction, the ECG may show ST-segment elevation myocardial infarction. Also, myocardial ischaemia may be exacerbated by acute aortic regurgitation, hypertension or hypotension, and shock in patients with or without pre-existing coronary artery disease. This may explain the observation that approximately 10% of patients presenting with acute Type B AD have ECG signs of myocardial ischaemia.147 Overall, comparisons of the incidence of myocardial ischaemia and infarction between the series and between Types A and -B aortic dissection are challenged by the lack of a common definition. In addition, the ECG diagnosis of non-transmural ischaemia may be difficult in this patient population because of concomitant left ventricular hypertrophy, which may be encountered in approximately one-quarter of patients with AD. If systematically assessed, troponin elevation may be found in up to 25% of patients admitted with Type A AD.143 Both troponin elevation and ECG abnormalities, which may fluctuate over time, may mislead the physician to the diagnosis of acute coronary syndromes and delay proper diagnosis and management of acute AD.
Congestive heart failure
in the setting of AD is commonly related to aortic regurgitation. Although more common in Type A AD, heart failure may also be encountered in patients with Type B AD, suggesting additional aetiologies of heart failure, such as myocardial ischaemia, pre-existing diastolic dysfunction, or uncontrolled hypertension. Registry data show that this complication occurs in <10% of cases of AD.131,145 Notably, in the setting of AD, patients with acute heart failure and cardiogenic shock present less frequently with the characteristic severe and abrupt chest pain, and this may delay diagnosis and treatment of AD. Hypotension and shock may result from aortic rupture, acute severe aortic regurgitation, extensive myocardial ischaemia, cardiac tamponade, pre-existing left ventricular dysfunction, or major blood loss.
Large pleural effusions
resulting from aortic bleeding into the mediastinum and pleural space are rare, because these patients usually do not survive up to arrival at hospital. Smaller pleural effusions may be detected in 15–20% of patients with AD, with almost equal distribution between Type A and Type B patterns, and are believed to be mainly the result of an inflammatory process.131,145
Pulmonary complications
of acute AD are rare, and include compression of the pulmonary artery and aortopulmonary fistula, leading to dyspnoea or unilateral pulmonary oedema, and acute aortic rupture into the lung with massive haemoptysis.
Syncope
is an important initial symptom of AD, occurring in approximately 15% of patients with Type A AD and in <5% of those presenting with Type B. This feature is associated with an increased risk of in-hospital mortality because it is often related to life-threatening complications, such as cardiac tamponade or supra-aortic vessel dissection. In patients with suspected AD presenting with syncope, clinicians must therefore actively search for these complications.
Neurological symptoms
may often be dramatic and dominate the clinical picture, masking the underlying condition. They may result from cerebral malperfusion, hypotension, distal thromboembolism, or peripheral nerve compression. The frequency of neurological symptoms in AD ranges from 15–40%, and in half of the cases they may be transient. Acute paraplegia, due to spinal ischaemia caused by occlusion of spinal arteries, is infrequently observed and may be painless and mislead to the Leriche syndrome.152 The most recent IRAD report on Type A AD described an incidence of major brain injury (i.e. coma and stroke) in <10% and ischaemic spinal cord damage in 1.0%.145 Upper or lower limb ischaemic neuropathy, caused by a malperfusion of the subclavian or femoral territories, is observed in approximately 10% of cases. Hoarseness, due to compression of the left recurrent laryngeal nerve, is rare.
Mesenteric ischaemia
occurs in <5% of patients with Type A AD.145 Adjacent structures and organs may become ischaemic as aortic branches are compromised, or may be affected by mechanical compression induced by the dissected aorta or aortic bleeding, leading to cardiac, neurological, pulmonary, visceral, and peripheral arterial complications. End-organ ischaemia may also result from the involvement of a major arterial orifice in the dissection process. The perfusion disturbance can be intermittent if caused by a dissection flap prolapse, or persistent in cases of obliteration of the organ arterial supply by FL expansion. Clinical manifestation is frequently insidious; the abdominal pain is often non-specific, patients may be painless in 40% of cases; consequently, the diagnosis is frequently too late to save the bowel and the patient. Therefore, it is essential to maintain a high degree of suspicion for mesenteric ischaemia in patients with acute AD and associated abdominal pain or increased lactate levels. The presence of mesenteric ischaemia deeply affects the management strategy and outcomes of patients with Type A AD; in the latest IRAD report, 50% of patients with mesenteric malperfusion did not receive surgical therapy, while the corresponding proportion in patients without this complication was 12%.145 In addition, the in-hospital mortality rate of patients with mesenteric malperfusion is almost three times as high as in patients without this complication (63 vs. 24%).145 Gastrointestinal bleeding is a rare but potentially lethal. Bleeding may be limited, as a result of mesenteric infarction, or massive, caused by an aorto-oesophageal fistula or FL rupture into the small bowel.
Renal failure
may be encountered at presentation or during hospital course in up to 20% of patients with acute Type A AD and in approximately 10% of patients with Type B AD.145 This may be the result of renal hypoperfusion or infarction, secondary to the involvement of the renal arteries in the AD, or may be due to prolonged hypotension. Serial testing of creatinine and monitoring of urine output are needed for an early detection of this condition.
6.3.4 Laboratory testing
In patients admitted to the hospital with chest pain and suspicion of AD, the following laboratory tests, listed in Table 5, are required for differential diagnosis or detection of complications.
Laboratory tests required for patients with acute aortic dissection
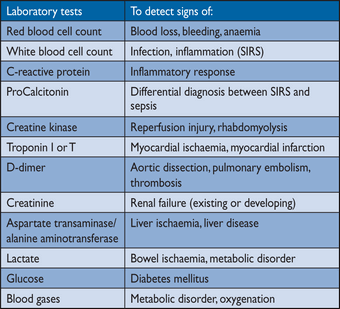 |
 |
SIRS = systemic inflammatory response syndrome.
Laboratory tests required for patients with acute aortic dissection
 |
 |
SIRS = systemic inflammatory response syndrome.
If D-dimers are elevated, the suspicion of AD is increased.153–159 Typically, the level of D-dimers is immediately very high, compared with other disorders in which the D-dimer level increases gradually. D-dimers yielded the highest diagnostic value during the first hour.153 If the D-dimers are negative, IMH and PAU may still be present; however, the advantage of the test is the increased alert for the differential diagnosis.
Since AD affects the medial wall of the aorta, several biomarkers have been developed that relate to injury of the vascular endothelial or smooth muscle cells (smooth muscle myosin), the vascular interstitium (calponin, matrix metalloproteinase 8), the elastic laminae (soluble elastin fragments) of the aorta, and signs of inflammation (tenascin-C) or thrombosis, which are in part tested at the moment but have not yet entered the clinical arena.159–162
6.3.5 Diagnostic imaging in acute aortic dissection
The main purpose of imaging in AAD is the comprehensive assessment of the entire aorta, including the aortic diameters, shape and extent of a dissection membrane, the involvement in a dissection process of the aortic valve, aortic branches, the relationship with adjacent structures, and the presence of mural thrombus (Table 6).153,163
Computed tomography, MRI, and TOE are equally reliable for confirming or excluding the diagnosis of AAD.78 However, CT and MRI have to be considered superior to TOE for the assessment of AAD extension and branch involvement, as well as for the diagnosis of IMH, PAU, and traumatic aortic lesions.82,164 In turn, TOE using Doppler is superior for imaging flow across tears and identifying their locations. Transoesophageal echocardiography may be of great interest in the very unstable patient, and can be used to monitor changes in-theatre and in post-operative intensive care.3
6.3.5.1 Echocardiography
The diagnosis of AD by standard transthoracic M-mode and two-dimensional echocardiography is based on detecting intimal flaps in the aorta. The sensitivity and specificity of TTE range from 77–80% and 93–96%, respectively, for the involvement of the ascending aorta.165–167 TTE is successful in detecting a distal dissection of the thoracic aorta in only 70% of patients.167
The tear is defined as a disruption of flap continuity, with fluttering of the ruptured intimal borders.150,168 Smaller intimal tears can be detected by colour Doppler, visualizing jets across the flap,169 which also identifies the spiral flow pattern within the descending aorta. Other criteria are complete obstruction of an FL, central displacement of intimal calcification, separation of intimal layers from the thrombus, and shearing of different wall layers during aortic pulsation.168
TTE is restricted in patients with abnormal chest wall configuration, narrow intercostal spaces, obesity, pulmonary emphysema, and in patients on mechanical ventilation.170 These limitations prevent adequate decision-making but the problems have been overcome by TOE.168,158 Intimal flaps can be detected, entry and re-entry tears localized, thrombus formation in the FL visualized and, using colour Doppler, antegrade and retrograde flow can be imaged while, using pulsed or continuous wave Doppler, pressure gradients between TL and FL can be estimated.169 Retrograde AD is identified by lack of-, reduced-, or reversed flow in the FL. Thrombus formation is often combined with slow flow and spontaneous contrast.150 Wide communications between the TL and FL result in extensive intimal flap movements which, in extreme cases, can lead to collapse of the TL, as a mechanism of malperfusion.151 Localized AD of the distal segment of the ascending aorta can be missed as it corresponds with the ‘blind spot’ in TOE.168
The sensitivity of TOE reaches 99%, with a specificity of 89%.168 The positive and negative predictive values are 89% and 99%, respectively, based on surgical and/or autopsy data that were independently confirmed.168,170 When the analysis was limited to patients who underwent surgery or autopsy, the sensitivity of TOE was only 89% and specificity 88%, with positive and negative predictive values at 97% and 93%, respectively.168
6.3.5.2 Computed tomography
The key finding on contrast-enhanced images is the intimal flap separating two lumens. The primary role of unenhanced acquisition is to detect medially displaced aortic calcifications or the intimal flap itself.171 Unenhanced images are also important for detecting IMH (see below).172,173
Diagnosis of AD can be made on transverse CT images, but multiplanar reconstruction images play an important complementary role in confirming the diagnosis and determining the extent of involvement, especially with regard to involvement of aortic branch vessels.174,175
The major role of multidetector CT is in providing specific, precise measurements of the extent of dissection, including length and diameter of the aorta, and the TL and FL, involvement of vital vasculature, and distance from the intimal tear to the vital vascular branches.176
The convex face of the intimal flap is usually towards the FL that surrounds the TL. The FL usually has slower flow and a larger diameter and may contain thrombi.176 In Type A AD, the FL is usually located along the right anterolateral wall of the ascending aorta and extends distally, in a spiral fashion, along the left posterolateral wall of the descending aorta. Slender linear areas of low attenuation may be observed in the FL, corresponding to incompletely dissected media, known as the ‘cobweb sign', a specific finding for identifying the FL. In most cases, the lumen that extends more caudally is the TL. Accurate discrimination between the FL and TL is important, to make clear which collaterals are perfused exclusively by the FL, as well as when endovascular therapy is considered.176
CT is the most commonly used imaging technique for evaluation of AAS, and for AD in particular,177–180 because of its speed, widespread availability, and excellent sensitivity of >95% for AD.177,179 Sensitivity and specificity for diagnosing arch vessel involvement are 93% and 98%, respectively, with an overall accuracy of 96%.177 Diagnostic findings include active contrast extravasation or high-attenuation haemorrhagic collections in the pleura, pericardium, or mediastinum.180
‘Triple-rule out’ is a relatively new term that describes an ECG-gated 64-detector CT study to evaluate patients with acute chest pain, in the emergency department, for three potential causes: AD, pulmonary embolism, and coronary artery disease. The inherent advantage of CT is its rapid investigation of life-threatening sources of acute chest pain, with a high negative predictive value.88,181 However, it is important to recognize highly mobile linear intraluminal filling defect, which may mimic an intimal flap on CT.182 The so-called ‘pulsation artefact’ is the most common cause of misdiagnosis.183 It is caused by pulsatile movement of the ascending aorta during the cardiac cycle between end-diastole and end-systole. The potential problem of pulsation artefacts can be eliminated with ECG-gating,77,183,184 or else by a 180° linear interpolation reconstruction algorithm.185 Dense contrast enhancement in the left brachiocephalic vein or superior vena cava, mediastinal clips, and indwelling catheters can all produce streak artefacts in the aorta, which may potentially simulate dissection. This difficulty can be avoided by careful attention to the volume and injection rate of intravenous contrast material administered.88
6.3.5.3 Magnetic resonance imaging
MRI is considered the leading technique for diagnosis of AD, with a reported sensitivity and specificity of 98%.164 It clearly demonstrates the extent of the disease and depicts the distal ascending aorta and the aortic arch in more detail than is achieved by TOE.186 The localization of entry and re-entry is nearly as accurate as with TOE and the sensitivity for both near to 90%.186 The identification of the intimal flap by MRI remains the key finding, usually seen first on spin-echo black-blood sequences.187 The TL shows signal void, whereas the FL shows higher signal intensity indicative of turbulent flow.188
MRI is also very useful for detecting the presence of pericardial effusion, aortic regurgitation, or carotid artery dissection.164,189 The proximal coronary arteries and their involvement in the dissecting process can be clearly delineated.190 Flow in the FL and TL can be quantified using phase contrast cine-MRI or by tagging techniques.191,192
Despite the excellent performance of this method, several methodological and practical limitations preclude the use of this modality in the majority of cases and in unstable patients.
6.3.5.4 Aortography
The angiographic diagnosis of AD is based upon ‘direct’ angiographic signs, such as the visualization of the intimal flap (a negative, frequently mobile, linear image) or the recognition of two separate lumens; or ‘indirect’ signs including aortic lumen contour irregularities, rigidity or compression, branch vessel abnormalities, thickening of the aortic walls, and aortic regurgitation.168 This technique is no longer used for the diagnosis of AD, except during coronary angiography or endovascular intervention.
6.3.6 Diagnostic work-up
The diagnostic work-up to confirm or to rule out AD is highly dependent on the a priori risk of this condition. The diagnostic tests can have different outputs according to the pre-test probability. In 2010, the ACC/American Heart Association (AHA) guidelines proposed a risk assessment tool based on three groups of information—predisposing conditions, pain features, and clinical examination—and proposed a scoring system that considered the number of these groups that were involved, from 0 (none) to 3 (Table 7).8 The IRAD reported the sensitivity of this approach, but a validation is not yet available.153 The presence of 0, 1, 2, or 3 groups of information is associated with increasing pre-test probability, which should be taken into account in the diagnostic approach to all AAS, as shown at the basis of the flow chart (Figure 6). The diagnostic flow chart combines the pre-test probabilities (Table 7) according to clinical data, and the laboratory and imaging tests, as should be done in clinical practice in emergency or chest pain units (Figure 6).
Clinical data useful to assess the a priori probability of acute aortic syndrome
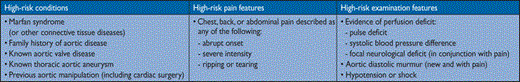 |
 |
Clinical data useful to assess the a priori probability of acute aortic syndrome
 |
 |
Recommendations on diagnostic work-up of acute aortic syndrome
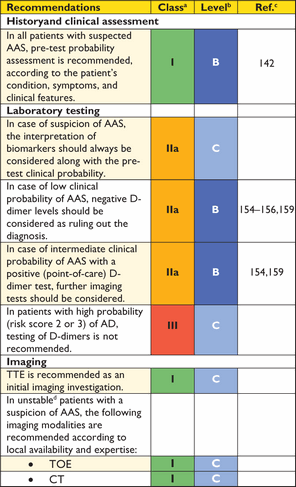 |
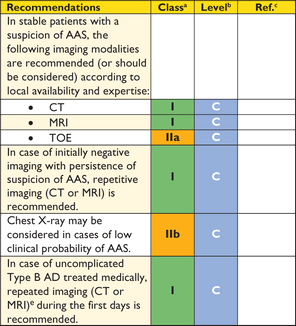 |
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dUnstable means very severe pain, tachycardia, tachypnoea, hypotension, cyanosis, and/or shock.
ePreferably MRI in young patients, to limit radiation exposure.
AAS = abdominal aortic aneurysm; AD = aortic dissection; CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
Recommendations on diagnostic work-up of acute aortic syndrome
 |
 |
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dUnstable means very severe pain, tachycardia, tachypnoea, hypotension, cyanosis, and/or shock.
ePreferably MRI in young patients, to limit radiation exposure.
AAS = abdominal aortic aneurysm; AD = aortic dissection; CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
6.3.7 Treatment
Whether or not the patient undergoes any intervention, medical therapy to control pain and the haemodynamic state is essential (see section 5.1).
6.3.7.1 Type A aortic dissection
Surgery is the treatment of choice. Acute Type A AD has a mortality of 50% within the first 48 hours if not operated. Despite improvements in surgical and anaesthetic techniques, perioperative mortality (25%) and neurological complications (18%) remain high.193,194 However, surgery reduces 1-month mortality from 90% to 30%. The advantage of surgery over conservative therapy is particularly obvious in the long-term follow-up.195
Based on that evidence, all patients with Type A AD should be sent for surgery; however, coma, shock secondary to pericardial tamponade, malperfusion of coronary or peripheral arteries, and stroke are important predictive factors for post-operative mortality. The superiority of surgery over conservative treatment has been reported, even in patients with unfavourable presentations and/or major comorbidities. In an analysis of 936 patients with Type A AD enrolled in the IRAD registry, up to the age of 80 years, in-hospital mortality was significantly lower after surgical management than with medical treatment. In octogenarians, in-hospital mortality was lower after surgery than with conservative treatment (37.9 vs. 55.2%); however, the difference failed to reach clinical significance, probably due to the limited sample size of participants over 80 years of age.196 While some have reported excellent surgical and quality-of-life outcomes in the elderly,197 others found a higher rate of post-operative neurological complications.198 Based on the current evidence, age per se should not be considered an exclusion criterion for surgical treatment.
For optimal repair of acute Type A AD in respect of long-term results—including risk of late death and late re-operation—the following points need to be addressed. In most cases of aortic insufficiency associated with acute Type A dissection, the aortic valve is essentially normal and can be preserved by applying an aortic valve-sparing repair of the aortic root.199–203 Alternatively, given the emergency situation, aortic valve replacement can be performed. In any case, it is preferable to replace the aortic root if the dissection involves at least one sinus of Valsalva, rather than perform a supracoronary ascending aorta replacement only. The latter is associated with late dilation of the aortic sinuses and recurrence of aortic regurgitation, and requires a high-risk re-operation.202,203 Various techniques exist for re-implantion of the coronary ostia or preservation of the ostia of the coronary arteries. A current topic of debate is the extent of aortic repair; ascending aortic replacement or hemiarch replacement alone is technically easier and effectively closes the entry site but leave a large part of the diseased aorta untreated. Patients with visceral or renal malperfusion in acute Type A AD often have their primary entry tear in the descending aorta. These patients might profit from extended therapies, such as ‘frozen elephant trunk’ repair in order to close the primary entry tear and decompress the TL. The importance of intraoperative aortoscopy and of immediate post-operative imaging—ideally in a hybrid suite—to reconfirm or exclude the effectiveness of therapy, is obvious. In contrast, more extensive repair, including graft replacement of the ascending aorta and aortic arch and integrated stent grafting of the descending aorta103,105 (‘frozen elephant trunk') as a one-stage procedure is technically more challenging and prolongs the operation, with an increased risk of neurological complications,204 but offers the advantage of a complete repair, with a low likelihood of late re-intervention.205 If the dissection progresses into the supra-aortic branches, rather than the classic ‘island’ technique, end-to-end grafting of all supra-aortic vessels may be considered, using individual grafts from the arch prosthesis.206–208
There is still controversy over whether surgery should be performed in patients with Type A AD presenting with neurological deficits or coma. Although commonly associated with a poor post-operative prognosis, recovery has been reported when rapid brain reperfusion is achieved,114,209 especially if the time between symptom onset and arrival at the operating room is <5 hours.210
One major factor influencing the operative outcome is the presence of mesenteric malperfusion at presentation. Malperfusion syndrome occurs in up to 30% of patients with acute AD. Visceral organ and/or limb ischaemia is caused by dynamic compression of the TL, due to high-pressure accumulation in the FL as the result of large proximal inflow into the thoracic aortic FL and insufficient outflow in the distal aorta. Malperfusion may also be caused by extension of the intimal flap into the organ/peripheral arteries, resulting in static ‘stenosis-like’ obstruction. In most cases, malperfusion is caused by a combination of dynamic and static obstruction; therefore, surgical/hybrid treatment should be considered for patients with organ malperfusion. Fenestration of the intimal flap is used in patients with dynamic malperfusion syndrome, to create a sufficient distal communication between the TL and FL to depressurize the FL. The classic technique comprises puncture of the intimal flap from the TL into the FL using a Brockenborough needle using a transfemoral approach.211,212 Puncture is performed at the level of the maximum compression of the TL in the abdominal aorta. Intravascular ultrasound may be useful to guide puncture of the FL.213 A 12–18 mm diameter balloon catheter is used to create one or several large communications between the two lumens. An alternative technique (the ‘scissor’ technique)214 for fenestration of the intimal flap is based on the insertion of two stiff guide wires, one in the TL and the other in the FL, through a single, transfemoral, 8 F sheath. The sheath is advanced over the two guide wires from the external iliac artery up to the visceral arteries, to create a large communication site.
Although performed with high technical success rates, fenestration alone may not completely resolve malperfusion. In a recent series, 75% of patients undergoing fenestration required additional endovascular interventions (e.g. stenting) for relief of ischaemia.215
Endovascular therapy alone, to treat Type A AD, has been attempted in highly selected cases but has not yet been validated.216,217
6.3.7.2 Treatment of Type B aortic dissection
The course of Type B AD is often uncomplicated so—in the absence of malperfusion or signs of (early) disease progression— the patient can be safely stabilized under medical therapy alone, to control pain and blood pressure.
Uncomplicated Type B aortic dissection:
Medical therapy
Patients with uncomplicated Type B AD receive medical therapy to control pain, heart rate, and blood pressure, with close surveillance to identify signs of disease progression and/or malperfusion (see section 5.1). Repetitive imaging is necessary, preferably with MRI or CT.
Thoracic endovascular aortic repair
Thoracic endovascular aortic repair (TEVAR) aims at stabilization of the dissected aorta, to prevent late complications by inducing aortic remodelling processes. Obliterating the proximal intimal tear by implantation of a membrane-covered stent-graft redirects blood flow to the TL, thus improving distal perfusion. Thrombosis of the FL results in shrinkage and conceptually prevents aneurysmal degeneration and, ultimately, its rupture over time. So far, there are few data comparing TEVAR with medical therapy in patients with uncomplicated Type B AD. The Investigation of Stent Grafts in Patients with Type B AD (INSTEAD) trial randomized a total of 140 patients with sub-acute (>14 days) uncomplicated Type B AD.218 Two-year follow-up results indicated that TEVAR is effective (aortic remodelling in 91.3% of TEVAR patients vs. 19.4% of patients receiving medical treatment; P < 0.001); however, TEVAR showed no clinical benefit over medical therapy (survival rates: 88.9 ± 3.7% with TEVAR vs. 95.6 ± 2.5% with optimal medical therapy; P = 0.15). Extended follow-up of this study (INSTEAD-XL) recently showed that aorta-related mortality (6.9 vs. 19.3%, respectively; P = 0.04) and disease progression (27.0 vs. 46.1%, respectively; P = 0.04) were significantly lower after 5 years in TEVAR patients compared with those receiving medical therapy only.219 No difference was found regarding total mortality. A similar observation has recently been reported from the IRAD registry, which, however, also included patients with complicated AD.220
Complicated Type B aortic dissection: endovascular therapy.
Thoracic endovascular aortic repair
Thoracic endovascular aortic repair (TEVAR) is the treatment of choice in complicated acute Type B AD.11 The objectives of TEVAR are the closure of the ‘primary’ entry tear and of perforation sites in the descending aorta. The blood flow is redirected into the TL, leading to improved distal perfusion by its decompression. This mechanism may resolve malperfusion of visceral or peripheral arteries. Thrombosis of the FL will also be promoted, which is the initiation for aortic remodelling and stabilization.
The term ‘complicated’ means persistent or recurrent pain, uncontrolled hypertension despite full medication, early aortic expansion, malperfusion, and signs of rupture (haemothorax, increasing periaortic and mediastinal haematoma). Additional factors, such as the FL diameter, the location of the primary entry site, and a retrograde component of the dissection into the aortic arch, are considered to significantly influence the patient's prognosis.221 Future studies will have to clarify whether these subgroups benefit from immediate TEVAR treatment.
In the absence of prospective, randomized trials, there is increasing evidence that TEVAR shows a significant advantage over open surgery in patients with acute complicated Type B AD. A prospective, multicentre, European registry including 50 patients demonstrated a 30-day mortality of 8% and stroke and spinal cord ischaemia of 8% and 2%, respectively.222
6.3.7.2.2.2. Surgery
Lower extremities artery disease, severe tortuosity of the iliac arteries, a sharp angulation of the aortic arch, and the absence of a proximal landing zone for the stent graft are factors that indicate open surgery for the treatment of acute complicated Type B AD. The aim of open surgical repair is to replace the descending aorta with a Dacron® prosthesis and to direct the blood flow into the TL of the downstream aorta by closing the FL at the distal anastomotic site, and to improve perfusion and TL decompression, which may resolve malperfusion.223
Owing to the fact that, in most patients, the proximal entry tear is located near to the origin of the left subclavian artery, the operation has to be performed in deep hypothermic circulatory arrest via a left thoracotomy. This surgical technique offers the possibility of an ‘open’ proximal anastomosis to the non-dissected distal aortic arch. Although the surgical results have improved over past decades, they remain sub-optimal, with in-hospital mortality ranging from 25–50%.224 Spinal cord ischaemia (6.8%), stroke (9%), mesenteric ischaemia/infarction (4.9%), and acute renal failure (19%) are complications associated with open surgery.225
Nowadays, surgery is rare in cases of complicated Type B AD, and has been replaced largely by endovascular therapy. For the most part, the aorta has to be operated in deep hypothermic circulatory arrest via a left posterolateral thoracotomy. Cross-clamping of the aorta, distal to the left subclavian artery, may be impractical in most cases because of the site of the entry tear, which is predominantly located near to the origin of the left subclavian artery. The aim of the surgical repair implies the resection of the primary entry tear and the replacement of the dissected descending aorta; as a consequence, the blood is directed into the TL, resulting in an improved perfusion and decompression of the TL in the thoraco-abdominal aorta. This mechanism may resolve malperfusion of visceral arteries and peripheral arteries. In particular clinical situations, the ‘frozen elephant trunk’ technique might also be considered in the treatment of complicated acute Type B AD without a proximal landing zone, as it also eliminates the risk of retrograde Type A AD.226
Recommendations for treatment of aortic dissection
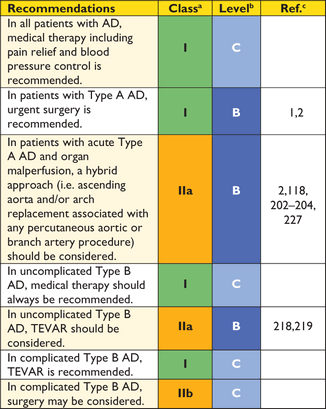 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
AD = aortic dissection; TEVAR = thoracic endovascular aortic repair.
Recommendations for treatment of aortic dissection
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
AD = aortic dissection; TEVAR = thoracic endovascular aortic repair.
6.4 Intramural haematoma
6.4.1 Definition
Aortic IMH is an entity within the spectrum of AAS, in which a haematoma develops in the media of the aortic wall in the absence of an FL and intimal tear. Intramural haematoma is diagnosed in the presence of a circular or crescent-shaped thickening of >5 mm of the aortic wall in the absence of detectable blood flow. This entity may account for 10–25% of AAS. The involvement of the ascending aorta and aortic arch (Type A) may account for 30% and 10% of cases, respectively, whereas it involves the descending thoracic aorta (Type B) in 60–70% of cases.228,229
6.4.2 Diagnosis
For the detection of an acute aortic IMH, TTE is inadequate because of its low sensitivity. For an IMH cut-off limit of 5 mm,230 the sensitivity of TTE for its detection is estimated to be lower than 40%. Based on these findings, TTE cannot be used as the sole imaging technique in patients with suspected AAS.231
CT and MRI are the leading techniques for diagnosis and classification of intramural haematoma. When evaluating the aorta using CT, an unenhanced acquisition is crucial for the diagnosis of IMH. A high-attenuation crescentric thickening of the aortic, extending in a longitudinal, non-spiral fashion, is the hallmark of this entity. In contrast to AD, the aortic lumen is rarely compromised in IMH, and no intimal flap or enhancement of the aortic wall is seen after administration of contrast. Using CT, the combination of an unenhanced acquisition followed by a contrast-enhanced acquisition yields a sensitivity as high as 96% for detection of IMH.232 Infrequently, however, the differentiation of IMH from atherosclerotic thickening of the aorta, thrombus, or thrombosed dissection may be difficult using CT. In those circumstances, MRI can be a valuable problem-solving tool, especially when dynamic cine gradient-echo sequences are applied.79,233,234 MRI may also provide a determination of the age of a haematoma, based on the signal characteristics of different degradation products of haemoglobin.88,187
In acute IMH Types A and B, imaging should always include a thorough attempt to localize a primary (micro) entry tear, which is very often present and therefore might lead the way to the choice of treatment, especially when considering TEVAR.
6.4.3 Natural history, morphological changes, and complications
The mortality rates of medically treated patients in European and American series are high,228,229,235–238 in contrast to Asian series.239,240 In the IRAD series, the in-hospital mortality of Type A IMH was similar to Type A AD, and related to its proximity to the aortic valve.229 On the other hand, several series showed that 30–40% of Type A IMH evolved into AD, with the greatest risk within the first 8 days after onset of symptoms.236 Acute Type B IMH has an in-hospital mortality risk of <10%, similar to that observed with descending Type B AD.228 Predictors of IMH complications in the acute phase are described in Table 8.
Overall, the long-term prognosis of patients with IMH is more favourable than that of patients with AD.247,248 However, survival at 5 years reported in IMH series ranged from 43–90%, depending on the population characteristics.178,228,236 Localized disruption, called ulcer-like projection (ULP) of the aorta, may appear within the first days or several months after the acute onset of symptoms (Web Figure 14), and this differs from PAU, which is related to atherosclerosis of the aortic wall.241,248 Although ULP has a poor prognosis in the ascending aorta,248 the course is more benign in Type B IMH.241,248 It appears that the greater the initial depth of the ULP, the greater the risk of associated complications.247,249,250
6.4.4 Indications for surgery and thoracic endovascular aortic repair
Therapeutic management in acute IMH should be similar to that for AD.
6.4.4.1 Type A intramural haematoma
Emergency surgery is indicated in complicated cases with pericardial effusion, periaortic haematoma, or large aneurysms, and urgent surgery (<24 hours after diagnosis) is required in most of Type A IMHs. In elderly patients or those with significant comorbidities, initial medical treatment with a ‘wait-and-watch strategy’ (optimal medical therapy with blood pressure and pain control and repetitive imaging) may be a reasonable option, particularly in the absence of aortic dilation (<50 mm) and IMH thickness <11 mm.239,240
6.4.4.2 Type B intramural haematoma
Medical treatment is the initial approach to this condition. Endovascular therapy or surgery would have the same indications as for Type B AD. The subgroup of patients with aortic dilation or ulcer-like projection (ULP) should be followed up closely and treated more aggressively if symptoms persist or reappear, or if progressive aortic dilation is observed.250 Indications for intervention (TEVAR rather than surgery) in the acute phase are an expansion of the IMH despite medical therapy, and the disruption of intimal tear on CT with contrast enhancement.
Recommendations on the management of intramural haematoma
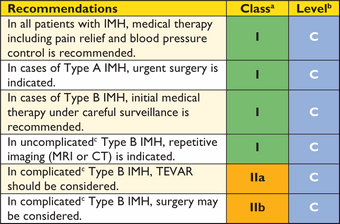 |
 |
aClass of recommendation.
bLevel of evidence.
cUncomplicated/complicated IMH means absence or present recurrent pain, expansion of the IMH, periaortic haematoma, intimal disruption.
CT = computed tomography; IMH = intramural haematoma; MRI = magnetic resonance imaging; TEVAR = thoracic endovascular aortic repair.
Recommendations on the management of intramural haematoma
 |
 |
aClass of recommendation.
bLevel of evidence.
cUncomplicated/complicated IMH means absence or present recurrent pain, expansion of the IMH, periaortic haematoma, intimal disruption.
CT = computed tomography; IMH = intramural haematoma; MRI = magnetic resonance imaging; TEVAR = thoracic endovascular aortic repair.
6.5 Penetrating aortic ulcer
6.5.1 Definition
Penetrating aortic ulcer (PAU) is defined as ulceration of an aortic atherosclerotic plaque penetrating through the internal elastic lamina into the media.251 Such lesions represent 2–7% of all AAS.252 Propagation of the ulcerative process may either lead to IMH, pseudoaneurysm, or even aortic rupture, or an acute AD.253 The natural history of this lesion is characterized by progressive aortic enlargement and development of saccular or fusiform aneurysms, which is particularly accelerated in the ascending aorta (Type A PAU).245,251,253,254 PAU is often encountered in the setting of extensive atherosclerosis of the thoracic aorta, may be multiple, and may vary greatly in size and depth within the vessel wall.255 The most common location of PAU is the middle and lower descending thoracic aorta (Type B PAU). Less frequently, PAUs are located in the aortic arch or abdominal aorta, while involvement of the ascending aorta is rare.245,251,256,257 Common features in patients affected by PAU include older age, male gender, tobacco smoking, hypertension, coronary artery disease, chronic obstructive pulmonary disease, and concurrent abdominal aneurysm.256–258 Symptoms may be similar to those of AD, although they occur more often in elderly patients and rarely manifest as signs of organ malperfusion.259 Symptoms have to be assumed to indicate an emergency as the adventitia is reached and aortic rupture expected. CT is the imaging modality of choice to diagnose PAU as an out-pouching of contrast media through a calcified plaque.
6.5.2 Diagnostic imaging
On unenhanced CT, PAU resembles an IMH. Contrast-enhanced CT, including axial and multiplanar reformations, is the technique of choice for diagnosis of PAU. The characteristic finding is localized ulceration, penetrating through the aortic intima into the aortic wall in the mid- to distal third of the descending thoracic aorta. Focal thickening or high attenuation of the adjacent aortic wall suggests associated IMH. A potential disadvantage of MRI in this setting, compared with CT, is its inability to reveal dislodgement of the intimal calcifications that frequently accompany PAU (Table 9).
Diagnostic value of different imaging modalities in acute aortic syndromes
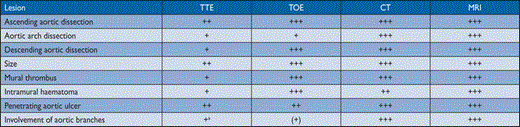 |
 |
aCan be improved when combined by vascular ultrasound (carotid, subclavian, vertebral, celiac, mesenteric and renal arteries).
++ + = excellent; ++ = moderate; += poor; (+) = poor and inconstant; CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
Diagnostic value of different imaging modalities in acute aortic syndromes
 |
 |
aCan be improved when combined by vascular ultrasound (carotid, subclavian, vertebral, celiac, mesenteric and renal arteries).
++ + = excellent; ++ = moderate; += poor; (+) = poor and inconstant; CT = computed tomography; MRI = magnetic resonance imaging; TOE = transoesophageal echocardiography; TTE = transthoracic echocardiography.
6.5.3 Management
In the presence of AAS related to PAU, the aim of treatment is to prevent aortic rupture and progression to acute AD. The indications for intervention include recurrent and refractory pain, as well as signs of contained rupture, such as rapidly growing aortic ulcer, associated periaortic haematoma, or pleural effusion.241,258,259
It has been suggested that asymptomatic PAUs with diameter >20 mm or neck >10 mm represent a higher risk for disease progression and may be candidates for early intervention.241 However, the size-related indications are not supported by other observations.253 The value of FDG-positron emission tomography/CT is currently being investigated, for the assessment of the degree and extension of lesion inflammation as a marker of aortic instability and potential guidance for therapy.86
6.5.4 Interventional therapy
In patients with PAU, no randomized studies are available that compare open surgical- and endovascular treatment. The choice of treatment is commonly based on anatomical features, clinical presentation, and comorbidities. Since these patients are often poor candidates for conventional surgery due to advanced age and related comorbidities—and the aortic lesions, due to their segmental nature, represent an ideal anatomical target for stenting—TEVAR is increasingly being used for this indication, with encouraging results.255,259–261
Recommendations on management of penetrating aortic ulcer
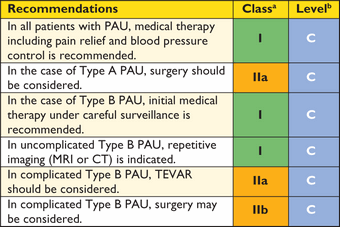 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; MRI = magnetic resonance imaging; PAU = penetrating aortic ulcer; TEVAR = thoracic endovascular aortic repair.
Recommendations on management of penetrating aortic ulcer
 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; MRI = magnetic resonance imaging; PAU = penetrating aortic ulcer; TEVAR = thoracic endovascular aortic repair.
6.6 Aortic pseudoaneurysm
Aortic pseudoaneurysm (false aneurysm) is defined as a dilation of the aorta due to disruption of all wall layers, which is only contained by the periaortic connective tissue. When the pressure of the aortic pseudoaneurysm exceeds the maximally tolerated wall tension of the surrounding tissue, fatal rupture occurs. Other life-threatening complications—due to the progressive increase of the size of the aortic pseundoaneurysm—include fistula formation and the compression or erosion of surrounding structures. Pseudoaneurysms of the thoracic aorta are commonly secondary to blunt thoracic trauma, as a consequence of rapid deceleration experienced in motor vehicle accidents, falls, and sports injuries.262 Iatrogenic aetiologies include aortic surgery and catheter-based interventions.263–265 Rarely, aortic pseudoaneurysms are secondary to aortic infections (mycotic aneurysms) and penetrating ulcers.
In patients with aortic pseudoaneurysms—if feasible and independently of size—interventional or open surgical interventions are always indicated. Currently, no randomized studies are available that compare outcomes after open surgical and endovascular treatment in aortic pseudoaneurysm patients. The choice of treatment is commonly based on anatomical features, clinical presentation, and comorbidities.
6.7 (Contained) rupture of aortic aneurysm
Contained rupture should be suspected in all patients presenting with acute pain, in whom imaging detects aortic aneurysm with preserved integrity of the aortic wall. In this setting, recurrent or refractory pain—as well as pleural or peritoneal effusions, particularly if increasing—identifies patients at highest risk of aortic rupture. At the time of imaging, aortic rupture may be difficult to differentiate from contained aortic rupture. In contrast to overt free rupture (in which disruption of all of the layers of the aortic wall leads to massive haematoma), in contained ruptures of aortic aneurysms (with or without pseudoaneurysm formation), perivascular haematoma is sealed off by periaortic structures, such as the pleura, pericardium and retroperitoneal space, as well as the surrounding organs. Therefore, patients with contained aortic rupture are haemodynamically stable.
6.7.1 Contained rupture of thoracic aortic aneurysm
6.7.1.1 Clinical presentation
Patients with contained rupture of a TAA usually present with acute onset of chest and/or back pain. Concurrent abdominal pain may be present in patients with symptomatic thoraco-abdominal aneurysms. Overt free aortic rupture typically leads rapidly to internal bleeding and death. Acute respiratory failure may be the result of free aortic rupture into the left hemithorax. Rarely, erosion into mediastinal structures can result in haemoptysis from aortobronchial fistula or haematemesis from an aorto-oesophageal fistula. The location of the rupture is of paramount importance, as it is pertinent to prognosis and management. As a general rule, the closer the location of the aneurysm to the aortic valve, the greater the risk of death. Fewer than half of all patients with rupture arrive at hospital alive; mortality may be as high as 54% at 6 hours and 76% at 24 hours after the initial event.123
6.7.1.2 Diagnostic work-up
With the suspicion of (contained) rupture of a TAA, CT is indicated, using a protocol including a non-contrast phase to detect IMH, followed by a contrast injection to delineate the presence of contrast leaks indicating rupture. In addition to the entire aorta, imaging should cover the iliac and femoral arteries, to provide sufficient information for the planning of surgical or endovascular treatment. Contained (also called impending) ruptures of TAA are indications for urgent treatment because of the risk of imminent internal bleeding and death. As a general rule and in the absence of contraindications, symptomatic patients should be treated regardless of the diameter of the aneurysm because of the risk of aortic rupture.266 Open surgical and endovascular options should be carefully balanced in terms of risks and benefits, case by case, depending also on local expertise. The planning and performance of TEVAR for (contained) rupture of TAA should be performed according to the recent ESC/European Association for Cardio-Thoracic Surgery consensus document.11 Favourable anatomical factors for an endovascular repair include the presence of adequate proximal and distal landing zones for the prosthesis and adequate iliac/femoral vessels for vascular access.
6.7.1.3 Treatment
Contained rupture of TAA is a condition requiring urgent treatment because, once overt free rupture occurs, most patients do not survive. Traditionally, this condition has been treated by open repair, but endovascular repair has emerged as an alternative treatment option for suitable patients. A meta-analysis of 28 retrospective series, comparing open with endovascular repair in a total of 224 patients, documented a 30-day mortality rate of 33% in the open surgical group and 19% in the TEVAR group (P = 0.016).267 In a retrospective multicentre analysis of 161 patients, the 30-mortalities in the surgical- and TEVAR groups were 25% and 17%, respectively (P = 0.26).268 The composite outcome of death, stroke, or permanent paraplegia occurred in 36% of patients in the open repair group, compared with 22% in the TEVAR group. An analysis of the US Nationwide Inpatient Sample data set identified 923 patients who underwent ruptured descending TAA repair between 2006 and 2008, and who had no concomitant aortic disorders. Of these patients, 61% underwent open repair and 39% TEVAR. Unadjusted in-hospital mortality was 29% for open surgery and 23% for TEVAR (P = 0.064).269 After multivariable adjustment, the odds of mortality, complications, and failure to rescue were similar for open surgery and TEVAR.
Recommendations for (contained) rupture the thoracic aortic aneurysm
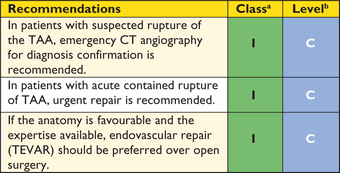 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; TAA = thoracic aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
Recommendations for (contained) rupture the thoracic aortic aneurysm
 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; TAA = thoracic aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
6.8 Traumatic aortic injury
6.8.1 Definition, epidemiology and classification
Blunt traumatic thoracic aortic injury (TAI) most often occurs as a consequence of sudden deceleration resulting from head-on or side-impact collisions, usually in high-speed motor vehicle accidents or falling from a great height. Rapid deceleration results in torsion and shearing forces at relatively immobile portions of the aorta, such as the aortic root or in proximity of the ligamentum arteriosum or the diaphragm. A combination of compression and upward thrust of the mediastinum, sudden blood pressure elevation, and stretching of the aorta over the spine may also explain the pathogenesis of TAI. Accordingly, TAI is located at the aortic isthmus in up to 90% of cases.270,271 A classification scheme for TAI has been proposed: Type I (intimal tear), Type II (IMH), Type III (pseudoaneurysm), and Type IV (rupture).272 Thoracic aortic injury is, after brain injury, the second most common cause of death in blunt trauma patients; the on-site mortality may exceed 80%. With improved rescue processes and rapid detection of TAI, patients who initially survive are more likely to undergo successful repair.
6.8.2 Patient presentation and diagnosis
The clinical presentation of TAI ranges from minor non-specific symptoms to mediastinal or interscapular pain. In a multicentre retrospective study of 640 patients a score data set was developed in one group and validated in another. Emergency CT should be performed. Computed tomography is quick and reproducible, with sensitivity and specificity close to 100% for TAI. Predictors of TAI were widened mediastinum, hypotension <90 mm Hg, long bone fracture, pulmonary contusion, left scapula fracture, haemothorax, and pelvic fracture. Sensitivity reached 93% and specificity 86% in the validation set of patients.273 Also, CT allows simultaneous imaging of other organs (brain, visceral and bones injuries). Other findings associated with TAI may include mediastinal haematoma, haemothorax, and at the level of the aortic wall pseudoaneurysm, intimal flap, or thrombus formation. Finally, CT allows for 3D reconstructions with MPR that are critical for TEVAR. Alternatively, TOE is widely available, relatively non-invasive, and can be performed quickly at the bedside or in the operating room. In a subset of 101 patients with TAI, TOE reached a sensitivity of 100% and a specificity of 98% for detection of an injury of the aortic wall, but was possible only in 93 (92%) patients. Traumatic aortic injury was found in 11 (12%) of 93 patients and validated by surgery or autopsy.274 In a smaller series of 32 patients, similarly high values were observed, yielding a sensitivity of 91% and a specificity of 100% for TAI with subadventitial injury. Only one intimal tear was missed.275 Despite these excellent results, TOE has a limited value in the evaluation of associated thoracic or abdominal injuries.
6.8.3 Indications for treatment in traumatic aortic injury
The appropriate timing of treatment in patients with TAI is still controversial. In haemodynamically stable patients, the majority of TAI-associated aortic ruptures were believed to occur within 24 hours. For this reason, immediate treatment of TAI has for many years been considered to be the standard of care. Subsequently, several studies have suggested a reduction in paraplegia and mortality associated with delayed aortic treatment in selected patients requiring management of additional extensive injuries.276 In those patients, aortic repair should then be performed as soon as possible after initial injury (i.e. within 24 hours). A classification system has recently been worked out.268
The type of aortic injury is a critical factor determining the timing of intervention. Patients with free aortic rupture or large periaortic haematoma should be treated as emergency cases. For all other conditions, the intervention may be delayed for up to 24 hours to allow for patient stabilization and the best possible conditions for the aortic intervention. An initial conservative management, with serial imaging, has been proposed for patients with minimal aortic injuries (intimal tear/Type I lesions), as most lesions remain stable or resolve.277,278
6.8.4 Medical therapy in traumatic aortic injury
In polytrauma patients, multidisciplinary management is vital to establish the correct timing of the interventions and treatment priorities. Aggressive fluid administration should be avoided because it may exacerbate bleeding, coagulopathy, and hypertension; to reduce the risk of aortic rupture, mean blood pressure should not exceed 80 mm Hg.272,279,280
6.8.5 Surgery in traumatic aortic injury
To facilitate access, open surgical repair of a TAI at the classic isthmus location requires exposure of the aorta via a left fourth interspace thoracotomy, as well as selective right lung ventilation. The aorta is clamped proximally to the origin of the left subclavian artery and distally to the injured segment. Until the mid-1980s, most of these procedures were completed with an expeditious clamp-and-sew technique. A meta-analyses of this technique reported mortality and paraplegia rates of 16–31% and 5–19%, respectively.262,281,282
Various methods of distal aortic perfusion have been used to protect the spinal cord. The use of extracorporeal circulation has been associated with a reduced risk of perioperative mortality and paraplegia. A meta-analysis and large cohort studies of active vs. passive perfusion showed a lower rate of post-operative paraplegia from 19% to 3% and a reduction in mortality from 30% to 12% associated with active perfusion.283,284
6.8.6 Endovascular therapy in traumatic aortic injury
Available data indicate that TEVAR, in suitable anatomies, should be the preferred treatment option in TAI.262,268,269,278,281,285–295 In a review of 139 studies (7768 patients), the majority being non-comparative case series, retrospective in design, and none being a randomized trial, a significantly lower mortality rate has been reported for TEVAR than for open surgery (9 vs. 19%; P < 0.01).276
Similarly, most other systematic reviews suggested an advantage from TEVAR, in terms of survival as well as a decreased incidence of paraplegia, when compared with open surgery. Endoleak rates of up to 5.2% and a stent collapse rate of 2.5%, with a mortality rate of 12.9% associated with the latter complication, have been reported for TEVAR.276,289
6.8.7 Long-term surveillance in traumatic aortic injury
CT is currently considered the standard imaging modality for follow-up in patients who benefit from TEVAR; however, given the frequent young age of patients with TAI, concerns arise with regard to cumulative exposure to radiation and iodinated contrast medium.83 For these reasons MRI is the best alternative for surveillance when magnetic resonance-compatible stent grafts are employed. It therefore seems rational to adopt a combination of a multiview chest X-ray and MRI, instead of CT, for long-term follow-up of these patients, with due consideration of the metallic composition of the endograft. By these two modalities, endoleaks, pseudoaneurysm, and stent graft material-related complications can be detected.
Recommendations for traumatic aortic injury
 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; TAI = traumatic aortic injury; TEVAR = thoracic endovascular aortic repair; TOE = transoesophageal echocardiography.
Recommendations for traumatic aortic injury
 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; TAI = traumatic aortic injury; TEVAR = thoracic endovascular aortic repair; TOE = transoesophageal echocardiography.
6.9 Iatrogenic aortic dissection
Iatrogenic aortic dissection (IAD) may occur in the setting of (i) catheter-based coronary procedures, (ii) cardiac surgery, (iii) as a complication of endovascular treatment of aortic coarctation,296,297 (iv) aortic endografting,298 (v) peripheral interventions, (vi) intra-aortic balloon counterpulsation and, more recently, (vii) during transcatheter aortic valve implantation.299 With respect to catheter-based coronary procedures, IAD is a rare complication, reported in less than 4 per 10 000 coronary angiographies and less than 2 per 1000 percutaneous coronary interventions.299–303 One series reported an incidence of 7.5 per 1000 coronary interventions.304 Iatrogenic AD can be induced when the catheter is pushed into the vessel wall during the introduction of a diagnostic or guiding catheter, and is usually located in the abdominal aorta. Iatrogenic AD can also be the result of retrograde extension into the ascending aorta of a vessel wall injury, most commonly located at the ostium of the right coronary artery, which is located along the right anterior convexity of the ascending aorta where dissections more easily extend upwards.300–304 Injury propagation may be favoured by contrast injections and extensive dissections involving the ascending aorta, the aortic arch, the supra-aortic vessels, and even the descending aorta may be observed. Furthermore, extension of the intimal flap towards the aortic valve may cause significant acute aortic regurgitation, haemopericardium and cardiac tamponade. Usually, the diagnosis of IAD is straightforward during angiography, characterized by stagnation of contrast medium at the level of the aortic root or ascending aorta. If needed, the extension of the process can be further investigated with TOE or CT. Clinical manifestations may range from the absence of symptoms to excruciating chest, back, or abdominal pain, according to the site of the AD. Hypotension, haemodynamic compromise, and shock may ensue. At times, the diagnosis of IAD may be difficult due to atypical presentation and relative lack of classic signs of dissection on imaging studies.305 The management of iatrogenic catheter-induced AD is not standardized. A conservative approach is frequently applied, especially for catheter-induced dissection of the abdominal aorta or iliac arteries, and for those located at the level of the coronary cusps. Whilst an IAD of the right coronary artery ostium may compromise flow at the ostium and require emergency coronary stenting, the outcome for the aortic wall is benign when the complication is promptly recognized and further injections are avoided. Treatment is conservative in most cases, with complete spontaneous healing observed in most instances. Rupture is exceedingly rare, but isolated reports of extensive secondary Type A dissections recommend careful monitoring of these patients. Dissections extending over several centimetres into the ascending aorta or further propagating do require emergency cardiac surgery.
The largest series, at a single high-volume centre, of iatrogenic catheter-based or surgically induced AD (n = 48) that underwent emergency surgical repair suggested a somewhat higher incidence following cardiac surgery than with coronary catheterization procedures.303 Early mortality was 42%, with no difference between catheter- or cardiac surgery-induced dissections. Iatrogenic AD during surgery occurred most frequently during aortic cannulation, insertion of the cardioplegia cannula, or manipulation of the aorta cross-clamp.303 In a report from IRAD, the mortality of Type A IAD (n = 34) was similar to that for spontaneous AD, while the mortality for iatrogenic Type B AD exceeded that during spontaneous AD.305 Several cases have been reported of IAD following transcatheter aortic valve implantations.299 The incidence of this complication is not known because, in large-scale registries and randomized trials, it is usually included in the endpoint ‘major vascular complications’ and is not reported separately.
7. Aortic aneurysms
Aneurysm is the second most frequent disease of the aorta after atherosclerosis. In these Guidelines, the management of aortic aneurysms is focused largely on the lesion, and is separated into TAAs and AAAs. This approach follows the usual dichotomy, in part related to the fact that different specialists tend to be involved in different locations of the disease. The pathways leading to TAA or AAA may also differ, although this issue has not been clearly investigated, and similarities between the two locations may outweigh disparities. Before presentation of the sections below, several points should be highlighted.
First, this dichotomy into TAA ad AAA is somehow artificial, not only because of the presence of thoraco-abdominal aneurysm, but also because of the possibility of tandem lesions. In a recent series, 27% of patients with AAA also presented a TAA, most of whom were women and the elderly.306 In another large study of more than 2000 patients with AAA, more than 20% had either synchronous or metachronous TAA.307 In a multicentre study screening for AAA during TTE, in those with AAA the ascending aorta was larger, with significantly higher rates of aortic valve disease (bicuspid aortic valve and/or grade 3 or more aortic regurgitation: 8.0 vs. 2.6% in those without AAA; P = 0.017).308 On the other hand, patients with AD are at risk of developing AAA, mostly unrelated to a dissected abdominal aorta.309 These data emphasize the importance of a full assessment of the aorta and the aortic valve in patients with aortic aneurysms, both at baseline and also during follow-up.
Second, the presence of aortic aneurysm may be associated with other locations of aneurysms. Iliac aneurysms are generally detected during aortic imaging, but other locations, such as popliteal aneurysms, may be missed. There are some discrepancies regarding the co-existence of peripheral aneurysms in patients with AAA, but a prevalence as high as 14% of either femoral or popliteal aneurysm has been reported.310 These locations are accessible for ultrasound imaging and should be considered in the general work-up of patients with AAA, along with screening for peripheral artery disease, a frequent comorbidity in this setting. Data on the co-existence of peripheral aneurysms in the case of TAA are scarce.
Third, patients with aortic aneurysm are at increased risk of cardiovascular events, mostly unrelated to the aneurysm, but plausibly related to common risk factors (e.g. smoking or hypertension) and pathways (e.g. inflammation), as well as the increased risk of cardiovascular comorbidities at the time of aneurysm diagnosis.311 Indeed, the 10-year risk of mortality from any other cardiovascular cause (e.g. myocardial infarction or stroke) may be as high as 15 times the risk of aorta-related death in patients with AAA.54 Even after successful repair, patients with TAA or AAA remain at increased risk for cardiovascular events.311 While no randomized, clinical trial (RCT) has yet specifically addressed the medical treatment of these patients to improve their general cardiovascular prognosis, it is common sense to advocate the implementation of general rules and treatments for secondary cardiovascular prevention, beyond specific therapies targeting the aneurysmal aorta as developed below.
Recommendations in patients with aortic aneurysm
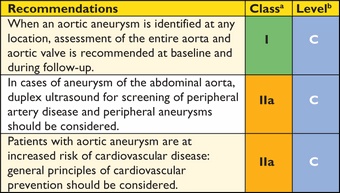 |
 |
aClass of recommendation.
bLevel of evidence.
Recommendations in patients with aortic aneurysm
 |
 |
aClass of recommendation.
bLevel of evidence.
7.1 Thoracic aortic aneurysms
TAA encompasses a wide range of locations and aetiologies, the most frequent being degenerative aneurysm of the ascending aorta.
7.1.1 Diagnosis
Patients with TAA are most often asymptomatic and the diagnosis is made following imaging, performed either for other investigative reasons or for screening purposes. The usefulness of screening patients at risk is well recognized in the case of Marfan syndrome. In patients with a BAV, the value of screening first-degree relatives is more debatable but can be considered.312 TAA is less frequently revealed by clinical signs of compression, chest pain, an aortic valve murmur, or during a complication (i.e. embolism, AD, or rupture).
7.1.2 Anatomy
In Marfan syndrome, aortic enlargement is generally maximal at the sinuses of Valsalva, responsible for annulo-aortic ectasia. This pattern is also seen in patients without Marfan phenotype. In patients with BAV, three enlargement patterns are described, according to whether the maximal aortic diameter is at the level of the sinuses of Valsalva, the supracoronary ascending aorta, or the sinotubular junction level (cylindrical shape). There is a relationship between the morphology of the ascending aorta and the valve fusion pattern.313
7.1.3 Evaluation
Once aortic dilation is suspected, based on echocardiography and/or chest X-ray, CT or MRI (with or without contrast) is required to adequately visualize the entire aorta and identify the affected parts. Key decisions regarding management of aortic aneurysms depend on their size. Hence, care must be taken to measure the diameter perpendicular to the longitudinal axis. A search should also be made for co-existing IMH, PAU, and branch vessel involvement of aneurysmal disease.
TTE, CT, and MRI should be performed with appropriate techniques and the consistency of their findings checked. This is of particular importance when diameters are borderline for the decision to proceed to intervention, and to assess enlargement rates during follow-up (see section 4). Follow-up modalities are detailed in section 13.
7.1.4 Natural history
Dimensions and growth rates of the normal aorta are described in section 3.
7.1.4.1 Aortic growth in familial thoracic aortic aneurysms
Familial TAAs grow faster, up to 2.1 mm/year (combined ascending and descending TAA). Syndromic TAA growth rates also vary. In patients with Marfan syndrome, the TAA growth is on average at 0.5–1 mm/year, whereas TAAs in patients with Loeys-Dietz syndrome (LDS) can grow even faster than 10 mm/year, resulting in death at a mean age of 26 years.85,314–316
7.1.4.2 Descending aortic growth
In general, TAAs of the descending aorta grow faster (at 3 mm/year) than those in ascending aorta (1 mm/year).317 In patients with Marfan syndrome with TAA, the mean growth rate after aortic valve and proximal aorta surgery for AD was 0.58 ± 0.5 mm/year for distal descending aortas. Dissection, urgent procedure, and hypertension were associated with larger distal aortic diameters at late follow-up and with more significant aortic growth over time.318
7.1.4.3 Risk of aortic dissection
There is a rapid increase in the risk of dissection or rupture when the aortic diameter is >60 mm for the ascending aorta and >70 mm for the descending aorta.266 Although dissection may occur in patients with a small aorta, the individual risk is very low.
7.1.5 Interventions
7.1.5.1 Ascending aortic aneurysms
Indications for surgery are based mainly on aortic diameter and derived from findings on natural history regarding the risk of complications weighed against the risk of elective surgery. Surgery should be performed in patients with Marfan syndrome, who have a maximal aortic diameter ≥50 mm.319 A lower threshold of 45 mm can be considered in patients with additional risk factors, including family history of dissection, size increase >3 mm/year (in repeated examinations using the same technique and confirmed by another technique), severe aortic regurgitation, or desire for pregnancy.312 Patients with Marfanoid manifestations due to connective tissue disease, without complete Marfan criteria, should be treated as Marfan patients. Earlier interventions have been proposed for aortic diameters >42 mm in patients with LDS.8 However, the underlying evidence is self-contradictory and the Task Force chose not to recommend a different threshold from Marfan syndrome.320,321 Patients with Ehlers-Danlos syndrome are exposed to a high risk of aortic complications, but no data are available to propose a specific threshold for intervention.
Surgery should be performed in patients with a BAV, who have a maximal aortic diameter ≥55 mm; these face a lower risk of complications than in Marfan.322 A lower threshold of 50 mm can be considered in patients with additional risk factors, such as family history, systemic hypertension, coarctation of the aorta, or increase in aortic diameter >3 mm/year, and also according to age, body size, comorbidities, and type of surgery. Regardless of aetiology, surgery should be performed in patients who have a maximal aortic diameter ≥55 mm.
The rate of enlargement, above which surgery should be considered, is a matter of debate. It should weigh prognostic implications against the accuracy of the measurements and their reproducibility. Rather than sticking to a given progression rate, it is necessary to rely on investigations performed using appropriate techniques with measurements taken at the same level of the aorta. This can be checked by analysing images and not just by considering the dimensions mentioned in the report. When rates of progression have an impact on the therapeutic decision, they should be assessed using alternative techniques (e.g. TTE and CT or MRI) and their consistency checked.
In borderline cases, the individual and family history, patient age, and the anticipated risk of the procedure should be taken into consideration. In patients with small body size, in particular in patients with Turner syndrome, an indexed aortic diameter of 27.5 mm/m2 body surface area should be considered.323 Lower thresholds of aortic diameters may also be considered in low-risk patients, if valve repair, performed in an experienced centre, is likely.34 In these borderline cases, decisions shared by the patient and the surgical team are important, following a thorough discussion regarding pros and contras for an earlier intervention, and a transparent presentation of surgical team's results.
For patients who have an indication for surgery on the aortic valve, lower thresholds can be used for concomitant aortic replacement (>45 mm) depending on age, body size, aetiology of valvular disease, and intraoperative shape and thickness of the ascending aorta. Surgical indications for aortic valve disease are addressed in specific guidelines.312 The choice between a total replacement of the ascending aorta—including the aortic root—by coronary re-implantation, and a segmental replacement of the aorta above the sinotubular junction, depends on the diameters at different sites of the aorta, in particular the sinuses of Valsalva. In cases of total replacement, the choice between a valve-sparing intervention and a composite graft with a valve prosthesis depends on the analysis of aortic valve function and anatomy, the size and site of TAA, life expectancy, desired anticoagulation status, and the experience of the surgical team.
7.1.5.2 Aortic arch aneuryms
Indications for surgical treatment of aneurysms of the aortic arch raise particular issues, due to the hazards relating to brain protection. In addition, few data exist on the natural history of isolated aortic arch aneurysms, since they are often associated with adjacent aneuryms of the ascending or descending aorta.
Surgery should be considered in patients who have an aortic arch aneurysm with a maximal diameter ≥55 mm or who present symptoms or signs of local compression. Decision-making should weigh the perioperative risks, since aortic arch replacement is associated with higher rates of mortality and stroke than in surgery of the ascending and descending aorta. Indications for partial or total aortic arch replacement are more frequently seen in patients who have an indication for surgery on an adjacent aneurysm of the ascending or descending aorta.
Arch vessel transposition (debranching) and TEVAR might be considered as an alternative to conventional surgery in certain clinical situations, especially when there is reluctance to expose patients to hypothermic circulatory arrest; however, especially after total arch vessel transposition, as well as in patients with the underlying diagnosis of acute Type B AD, the risk of retrograde Type A AD as a direct consequence of the procedure is elevated and should be weighed against the remaining risk of conventional surgery.105,117,324,325
7.1.5.3 Descending aortic aneurysms
The treatment of descending aortic aneurysms has been re-orientated with the development of TEVAR using stent grafts. No randomized trials exist to guide the choice between open surgery and TEVAR. From non-randomized comparisons and meta-analyses, early mortality is lower after TEVAR than open surgery.326–330 Early mortality depends on the extent of repair and patient characteristics, in particular age and comorbidities. Overall mid-term survival does not differ between TEVAR and surgery.327,328 During follow-up, there is a contrast between low mortality related to aortic complications and relatively high overall mortality, especially from cardiopulmonary causes.331,332
TEVAR should be considered in patients who have a descending TAA with a maximal diameter ≥55 mm. When surgery is the only option, it should be considered in patients with a maximal diameter ≥60 mm. Lower thresholds can be considered in patients with Marfan syndrome. Indications for treatment and the choice between TEVAR and open surgery should be made by a multidisciplinary team with expertise in both methods, taking into consideration patient age, comorbidities, and life expectancy, and conducting a thorough analysis of the arterial tree to assess the feasibility and presumed risks of each technique: extent and size of aneurysm, associated atheroma, collaterals, and size and length of the landing zone for endovascular grafting and vascular access.11,333 The lack of information on long-term results of TEVAR should be kept in mind, in particular in young patients. Surgery and TEVAR may be combined in hybrid approaches.
In cases of Marfan disease, surgery should be preferred over TEVAR. There is no evidence supporting any use of TEVAR in patients with connective tissue disease, except in emergency situations in order to get initial stabilization as a bridge to definitive surgical therapy.334,335
Recommendations on interventions on ascending aortic aneurysms
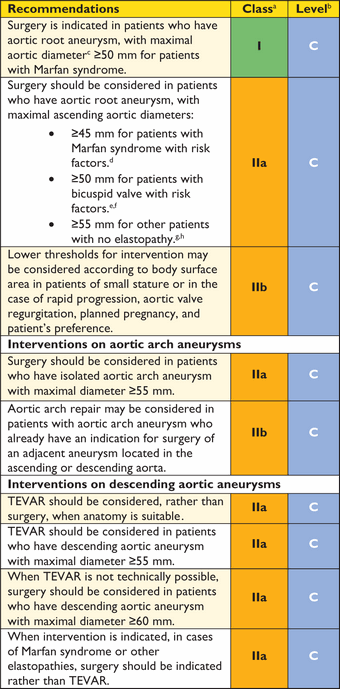 |
 |
aClass of recommendation.
bLevel of evidence.
cDecision should also take into account the shape of the different parts of the aorta. Lower thresholds can be used for combining surgery on the ascending aorta for patients who have an indication for surgery on the aortic valve.
dFamily history of AD and/or aortic size increase >3 mm/year (on repeated measurements using the same imaging technique, at the same aorta level, with side-by-side comparison and confirmed by another technique), severe aortic or mitral regurgitation, or desire for pregnancy.
eCoarctation of the aorta, systemic hypertension, family history of dissection, or increase in aortic diameter >3 mm/year (on repeated measurements using the same imaging technique, measured at the same aorta level, with side-by-side comparison and confirmed by another technique).
fPending comorbidities in the elderly.
gSee text in section 8.
hFor patients with LDS or vascular type IV Ehlers-Danlos syndrome (EDS), lower thresholds should be considered, possibly even lower than in Marfan syndrome. There are no data to provide figures and a sensible case-by-case approach is the only option.
Recommendations on interventions on ascending aortic aneurysms
 |
 |
aClass of recommendation.
bLevel of evidence.
cDecision should also take into account the shape of the different parts of the aorta. Lower thresholds can be used for combining surgery on the ascending aorta for patients who have an indication for surgery on the aortic valve.
dFamily history of AD and/or aortic size increase >3 mm/year (on repeated measurements using the same imaging technique, at the same aorta level, with side-by-side comparison and confirmed by another technique), severe aortic or mitral regurgitation, or desire for pregnancy.
eCoarctation of the aorta, systemic hypertension, family history of dissection, or increase in aortic diameter >3 mm/year (on repeated measurements using the same imaging technique, measured at the same aorta level, with side-by-side comparison and confirmed by another technique).
fPending comorbidities in the elderly.
gSee text in section 8.
hFor patients with LDS or vascular type IV Ehlers-Danlos syndrome (EDS), lower thresholds should be considered, possibly even lower than in Marfan syndrome. There are no data to provide figures and a sensible case-by-case approach is the only option.
7.2 Abdominal aortic aneurysm
7.2.1 Definition
While an aneurysm is generally defined as arterial enlargement with loss of arterial wall parallelism, AAA—almost exclusively infrarenal—is usually defined as a diameter ≥30 mm. Several authors proposed an alternative definition of a >50% increased diameter, but this cannot always be determined, especially when the limit between the aneurysmal and disease-free zones is not well delineated. The main aetiology of this disease is degenerative, although it is frequently associated with atherosclerotic disease.
7.2.2 Risk factors
Age, male gender, personal history of atherosclerotic cardiovascular disease, smoking and hypertension are all associated with the presence of AAA.336 Dyslipidaemia is considered as a weaker risk factor while, in contrast, diabetic patients are at decreased risk for AAA.336 A family history of AAA is a powerful predictor of prevalent AAA and risk for the condition increases exponentially with the number of siblings affected.336–338,339
7.2.3 Natural history
Large and life-threatening AAA is preceded by a long period of subclinical growth in the diameter of the aneurysm, estimated at <1–6 mm/year.95,340 These average rates cover a wide range of variability in diameter progression, which may depend on genetic and environmental factors—among which continued smoking is the most potent factor for a rapid growth. Also, the larger the AAA, the higher its growth rate.340 The risk of rupture rises exponentially with the aneurysm's maximal diameter and is higher in women than in men at similar diameters; women present ruptured AAA on average 10 mm smaller than men.
7.2.4 Diagnosis
7.2.4.1 Presentation
Before its cataclysmic presentation when ruptured, AAA is mostly silent. The most frequent mode of detection is incidental, during abdominal imaging for any indication. Atypical abdominal or back pain may be present but should not be awaited in order to reach a diagnosis. Systematic palpation of the abdomen during cardiovascular examination may detect a pulsatile abdominal mass, but its sensitivity is poor. Acute abdominal pain and shock are usually present in the case of ruptured AAA, sometimes preceded by a less intense abdominal pain for contained rupture.
7.2.4.2 Diagnostic imaging
Ultrasonography is an excellent tool for screening and surveillance, without risk and at low cost. Diameter measurements should be performed in the plane perpendicular to the arterial axis, to avoid any overestimation of the actual diameter (see section 4).
Considered the ‘gold standard’ in the past, aortography enabled optimal imaging of the length of the aorto-iliac lesion, the collateral or variant anatomy, the location and severity of occlusive disease, and the associated aneurysms in the visceral or iliac arteries. Its limitations are high radiation dose, contrast load, and its invasive nature. Also, this technique does not provide information about thrombus or the aneurysmal sac, and may misjudge the aortic diameter.
Because of technical improvements, their relatively non-invasive nature and lower cost, CT and MRI have emerged as the current ‘gold standards’ in the pre-operative and post-operative evaluation of AAAs. Operator proficiency and availability of equipment may determine the preferred modality. Computed tomography accurately visualizes the aorto-iliac lesions, including calcifications, but requires ionizing radiation and iodinated contrast. Breath-held dynamic contrast-enhanced MRI allows rapid acquisition of images in any plane, independent of flow. Its disadvantages include non-visualization of calcifications and the usual contraindications (e.g. metal implants).
The pre-operative assessment of AAAs includes the measurement of their maximal transverse perpendicular diameter and the relationship of the aneurysm to the renal arteries (Web Figure 15). Their lengths, as well as diameters, angulations, and tortuosity, are particularly important for endovascular aneurysm repair at the level of the segment of normal calibre of the aorta, below the renal arteries (‘proximal neck’) and the iliac arteries (‘distal neck’). Pre-operative imaging also reveals iliac or hypogastric aneurysms, occlusive disease in the iliac or renal arteries, and the presence of vascular abnormalities.
7.2.4.3 Screening abdominal aortic aneurysm in high-risk populations
The grim prognosis of ruptured AAA (mortality >60–70%) contrasts with the excellent survival rate (>95%) after planned AAA operation. This observation, along with the silent course of AAA and the possibility of detecting it easily with ultrasound, led to the consideration of mass screening in subgroups at risk (i.e. men ≥65 years, smokers, and those with a family history of AAA). Using abdominal echography, four randomized trials (>125 000 participants; three exclusively in men) compared the outcomes of population-based studies with or without AAA screening. The prevalence of AAA in these studies was on average 5.5%. Overall, AAA screening in men >65 years was associated with a significant 45% decreased risk of AAA-related mortality at 10 years, with a borderline 2% total decrease in risk of mortality (P = 0.05).341 Few (∼9300) women were included, confined to one trial, and showed no benefit from ultrasound screening.
Based on these trials, population-wide AAA screening programmes are currently proposed in several countries,342 with mixed results owing to difficulties over implementation.343 Several countries have not implemented such a programme, despite national guidelines in favour of AAA screening.342 Indeed, some doubts have been cast over the good results of the trials performed during the 1990s, since the epidemiology of AAA is evolving, with decreased rates of the incidence of AAA attributed largely to the decreasing rates of smoking in western countries. In a recent cohort of Swedish men >65 years of age, the prevalence of AAA was estimated at 2.2%.344
In the absence of a systematic population-screening programme, opportunistic screening may be an alternative for the detection of AAA. Indeed, in a series of patients with ruptured AAA who were managed in Scotland, three-quarters were unaware of having an AAA before rupture, even though three-quarters of the entire study population had attended a medical facility in the preceding 5 years.345 Opportunistic screening is defined here as the use of ultrasound to detect AAA (while abdominal imaging is not specifically planned) in situations where both the ultrasound machines and expertise are easily accessible. The most appealing situation for cardiologists is during echocardiography, since abdominal aorta imaging can be performed using the same probe. Several single-centre studies reported detection of AAAs during TTE in 0.8–6.0% of cases, with discrepancies related to inclusion and definition criteria, as well as specific factors inherent to each centre.346 In a recent nationwide survey in France, the prevalence of AAA screened immediately after TTE was 3.7%, at a low extra cost related to the time necessary for screening.347
7.2.5 Management of small abdominal aortic aneurysms
The definition of ‘small’ AAA varies in the literature, being usually either 30–49 mm or 30–54 mm, the upper limit depending on the threshold set for intervention; however, the AAA diameter cannot be considered as the sole criterion for the decision to intervene.
In this document, ‘small’ AAA encompasses situations where endovascular or surgical intervention is not yet considered. Indeed, two trials, the Aneurysm Detection And Management (ADAM) and the UK Small Aneurysm Trial (UKSAT) compared the benefits of early surgery for AAAs of 40–55 mm diameter against a surveillance strategy.348,349 A recent meta-analysis of these two trials demonstrated an early survival benefit in the surveillance group (due to the mortality in the surgery arm) without significant differences in long-term survival (6-year mortality: odds ratio (OR) 1.11; 95% confidence interval [CI] 0.91–1.34).350 In line with these trials, the Comparison of surveillance vs. Aortic Endografting for Small Aneurysm Repair showed no benefits from early EVAR in AAAs of 41–54 mm diameter, compared with the surveillance strategy combining regular imaging and prompt intervention in cases of predefined criteria (symptoms, or AAA >55 mm or enlargement >10 mm/year).351 However, the management of these patients should not be limited to a strategy of ‘watchful waiting’: they are at higher risk by far of dying from major cardiovascular events (e.g. myocardial infarction) than from AAA rupture. The participants in the Cardiovascular Health Study with an AAA >30 mm had a 10-year risk of fatal myocardial infarction of 38%, compared with an AAA-related mortality of 2%.54 Accordingly, in the UK Small Aneurysm Trial, aneurysmal diameter was an independent predictor of cardiovascular mortality (hazard ratio 1.34 and 1.31 for every 8 mm enlargement during surveillance and after surgery, respectively). Hence, medical therapy in small AAAs presents three objectives: to prevent cardiovascular events, to limit AAA growth, and to prepare the patient optimally in order to reduce perioperative risk once intervention is indicated. These patients should be categorized as at high risk, so all of the usual actions for secondary prevention can be applied, although no specific trial on patients with small AAAs has ever been undertaken. The measures addressed below will focus only on actions to specifically reduce the AAA rate of growth, but they are all useful for achieving the other two aforementioned objectives.
7.2.5.1 Management of risk factors
In a recent meta-analysis using data from 15 475 patients with AAA >30 mm, current smoking was associated with an increased rate of expansion of 0.35 mm/year, which is twice as fast as AAA growth in previous- or non-smokers.352 Similarly, data from population-based studies indicated that tobacco smoking was the most important predictor of future aortic aneurysm outcomes.353
There is no evidence of any beneficial effect on AAA growth from diet intervention or exercise prescription, but both are reasonable in patients at high risk of AAA. In a recent trial involving 140 patients with small (<55 mm) AAAs, in-house and home training over 3 years led to improved cardiopulmonary fitness, without any greater rate of enlargement than in the usual care arm.354 Intense isometric exercise is usually discouraged.
7.2.5.2 Medical therapy
Several small studies of unequal quality have assessed different drug classes with a view to reducing AAA growth, hypothetically by reducing either the wall shear stress or the inflammation, both of which play key roles in growth of AAAs. A meta-analysis355 of these studies led to the following results: while cohort studies suggested potential benefits of beta-blockers (pooled growth rate difference –0.62 mm/year; 95% CI –1.00 to –0.24) this finding was not confirmed in three RCTs (pooled growth rate difference –0.05 mm/year; 95% CI –0.16 to 0.05). The results of another meta-analysis were consistent with these findings.356 Two cohort studies suggested that statins were beneficial (pooled growth rate difference of –2.97; 95% CI –5.83 to –0.11), consistent with another meta-analysis of five longitudinal series.357 Doxycycline and roxithromycin have been evaluated in two RCTs without significant benefits (pooled growth rate difference –1.32 mm/year; 95% CI –2.89 to 0.25). Regarding ACE-inhibitors, a large population-based case-control study suggested a beneficial effect for this therapeutic class to prevent rupture (odds ratio 0.82; 95% CI 0.74–0.90), while this association was not found with other hypertensive drugs, including beta-blockers.358 Recently two studies provided mutually contradictory results: while the use of ACE-inhibitors was associated with increased AAA growth in UKSAT (the trial was not designed to assess this therapy),352 the Chichester study suggested beneficial effects of renin-angiotensin inhibitors, with significant results for those on angiotensin receptors blockers.359 Overall, these data require further investigation in well-designed, large RCTs; however, both statins and ACE-inhibitors should also be considered in these patients, to reduce risk of cardiovascular disease. According to the latest ESC Guidelines on hypertension in 2013, beta-blockers should be included as a first-line treatment for patients with hypertension and AAA.82
Enlargement of an AAA is usually associated with the development of an intraluminal mural thrombus. The presence, development, and rupture of aneurysms have been related to thrombus size, so that the use of antiplatelet therapy has been suggested to reduce complication rates in AAA.360 In the absence of any RCT, several cohort studies have analysed the potential benefits of aspirin in patients with AAA, especially in those in whom the lesion is large enough for the development of mural thrombus. In the Viborg study,361 the perioperative risk was more than twice as high in non-users of aspirin vs. users, even after adjustment for smoking and comorbidities. In a Swedish study,362 the concomitant use of aspirin and statins was significantly associated with the lowest rates of AAA growth. In contrast, a secondary analysis of UKSAT,363 as well as another study,364 did not find any significant difference in terms of AAA growth between aspirin users and non-users. Overall, data on the benefits of aspirin in reducing AAA growth are contradictory; however, most patients with AAAs are at increased risk of non-AAA-related cardiovascular events. Given the strong association between AAA and other atherosclerotic diseases, the use of aspirin may be considered according to the presence of other cardiovascular comorbidities.
The analysis of the RESCAN collaborative study is awaited, to provide insights regarding the benefits of these different drug classes in slowing AAA growth.365
7.2.5.3 Follow-up of small abdominal aortic aneurysm
Several studies have attempted to address the optimal pace for ultrasound surveillance of small AAAs. After a first imaging of the abdominal aorta, those with an aorta diameter <25 mm can be considered to be at very low risk of large AAA within the following 10 years,54 while an initial aorta of 26–29 mm merits a new assessment after 4 years.54,366 During the 13-year follow-up of participants in the Multicentre Aneurysm Screening Study (MASS), half of the ruptured AAAs had a baseline aortic diameter within the 25–29 mm range.367 Based on a recent individual-based meta-analysis of trials and observational studies with repeated AAA measurements over time, intervals of 3, 2, and 1 year(s) can be safely proposed for AAAs of 30–39, 40–44 and 45–54 mm diameter, respectively, with a risk <1% of rupture in men.365 In the same report, women experienced similar growth rates but a fourfold increased risk of rupture. Web Table 2 presents the average growth, risk of surgery, and risk of rupture in men and women according to AAA diameter. Women with 45 mm AAA had a risk of rupture equivalent to men with a 55 mm AAA, so a lower intervention threshold, rather than shorter intervals of follow-up, may be considered.
Recommendations for abdominal aortic aneurysm screening
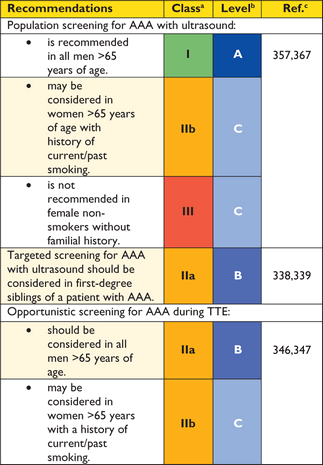 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
AAA = abdominal aortic aneurysm; TTE = transthoracic echocardiography.
Recommendations for abdominal aortic aneurysm screening
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
AAA = abdominal aortic aneurysm; TTE = transthoracic echocardiography.
7.2.6 Abdominal aortic aneurysm repair
7.2.6.1 Pre-operative cardiovascular evaluation
Coronary artery disease is the leading cause of early mortality after surgery for AAA. Angiographic evidence of coronary artery disease can be found in approximately two-thirds of patients with AAA, of which one-third are asymptomatic.336,367,368 The long duration of AAA repair procedures, the need for aortic clamping, and physiological stress from blood loss and fluid shifts may be strong triggers for acute ischaemic events. Thus, open repair of AAA is associated with a high risk (>5%) for perioperative cardiovascular complications (death, myocardial infarction, stroke).369 Endovascular AAA repair procedures, however, carry a lower risk (1–5%) than open surgery.370 The need for—and clinical value of—pre-operative risk stratification before repair of AAA depends on the risk of the procedure (i.e. open vs. endovascular repair) and clinical, patient-specific risk factors.371 For a more detailed description of risk stratification algorithms, the reader is referred to the recently updated ESC Guidelines.372
7.2.6.2 Aortic repair in asymptomatic abdominal aortic aneurysm
The management of AAA depends on aneurysm diameter. The indication for AAA repair needs to balance the risk of aneurysm surveillance and the associated risk of rupture against the surgical risk at a certain threshold diameter. Today, periodic ultrasound surveillance of the aneurysm—until it reaches 55 mm or becomes symptomatic or fast growing (>10 mm/year)—is regarded as a safe strategy for patients with small AAAs. This is based on the findings of two large multicentre RCTs (UKSAT and ADAM), both launched in the early 1990s.348,373 Few women were included in these trials and neither had the power to detect differences in all-cause mortality in this specific subgroup; however, there is evidence that women are more likely to rupture under surveillance and tend to suffer AAA rupture at a smaller aortic diameter than men.348,365,374 Even though evidence for threshold diameter in women is scarce, intervention at a smaller diameter (>50 mm) may be justified.
7.2.6.3 Open aortic aneurysm repair
Since its first use by Dubost et al. in the early 1950s, open AAA repair has been regarded as the default surgical intervention for AAA,375 but it carries a certain risk of mortality and morbidity, particularly in terms of cardiovascular events. Operative mortality from elective open surgical AAA repair was estimated in a variety of studies, but the figures vary considerably between centres and countries—relating to the type and design of the study—and range from 1% (selected centres of excellence) to 8% (population-based cohorts).376 There is even a discrepancy in quoted surgical mortality between different RCTs. For instance, the UKSAT and the ADAM trial quoted 30-day mortality rates of 5.6% and 2.7%, respectively, but it must be remembered that both trials included all AAAs, irrespective of anatomy, unless renal artery re-implantation was expected.348,373 A review combining results from 64 studies found an average mortality rate of 5.5%.377
Patient fitness is an important predictor and many authors tried to estimate the individual patient operative risk in order to identify subsets at different risk levels. The presence of cardiac and respiratory diseases as well as impaired renal function increases perioperative mortality of elective open AAA repair, whilst the impact of age as an independent factor is controversial.378,379 Other predictors of outcome are operator experience and hospital volume as discussed elsewhere in this document.
Outcomes of open ruptured AAA repair are much worse than those for elective AAA repair, and again results vary substantially across centres and countries. Bown et al. combined the results from 171 studies in a meta-analysis to determine the outcomes of ruptured AAA.380 The pooled estimate of operative mortality rate was 48%, although single centres report prospectively collected mortality results as low as 15%.381 A meta-regression analysis accounting for date of each study showed a 3.5% reduction in operative mortality per decade, whereas the intraoperative mortality rate remained stable at 15%, suggesting that overall improvements in outcome were not due to surgery-related factors.380
7.2.6.4 Endovascular aortic aneurysm repair
Endovascular aortic aneurysm repair was introduced in the early 1990s. The greatest advantage of EVAR is in its less invasive nature, which allows a shorter post-operative convalescence time. A meta-analysis of 161 studies reported a pooled operative mortality rate of 3.3% (95% CI 2.9–3.6); however, results have improved rapidly over time with lower mortality rates, at 1.4%, in recent studies.382
On the other hand, the long-term efficacy of EVAR remains a matter of concern. Subsequent lifelong imaging surveillance is currently required to monitor for late complications, including endoleaks, migration, and rupture. Late complications, including secondary sac ruptures, are closely linked to aortic sac enlargement over time. A recent study evaluated current compliance with anatomical guidelines for EVAR and the relationship between baseline aorto-iliac arterial anatomy and post-EVAR sac enlargement. This study from the USA showed that the incidence of AAA sac enlargement >5 mm after EVAR was 41% at 5 years and this rate increased over the study period, probably due to a more liberal use of EVAR outside the indication for use.383
The key feature of EVAR is the fluoroscopically guided insertion of an endograft through the femoral arteries, in order to re-line the aorta. Its feasibility depends on multiple factors, including aortic anatomy, individual clinical judgment, and manufacturers' guidelines. The proportion of AAAs suitable for EVAR varies between different studies, ranging from 15–68%.384 A recent study involving 241 patients and three different devices showed an overall 49.4% suitability rate for EVAR. Its authors assumed that the use of newer, low-profile devices would allow for EVAR in up to 60% of the AAA cases.385
7.2.6.5 Comparative considerations of abdominal aortic aneurysm management
Endovascular aortic repair is a valid alternative to surgical repair of AAA; however, in patients with more complex aortic anatomy—including those with aneurysms in close proximity to- or involving the renal arteries, who are unsuitable for EVAR—open repair remains the standard. Endovascular treatment strategies exist to address such aneurysms, for instance branched or fenestrated endografts, but comparisons with open repair in RCTs are still awaited.
For a subset of AAA patients, all being anatomically and physiologically eligible for both conventional EVAR and open repair, a head-to-head comparison of the two techniques was prompted in the late 1990s. The first and largest RCT comparing open with endovascular repair for large AAA started in the United Kingdom in 1999, the UK EndoVascular Aneurysm Repair (EVAR)-1 trial.386–388 Similar trials followed in the Netherlands: the Dutch Randomized Aneurysm Management (DREAM) trial.389–391 In the Unites States, there was the Open Vs. Endovascular Repair (OVER) trial;392,393 and in France, the Anévrisme de l'aorte abdominale: Chirurgie vs. Endoprothèse trial.394 The results of all these, including two smaller trials from Canada and the Netherlands,395,396 were combined in a recent meta-analysis resulting in 1470 patients allocated to EVAR and 1429 allocated to open repair.397 The trials reported different follow-up periods, with only the EVAR-1 and DREAM trials reporting longer-term follow-up (>6 years). Short-term (30 day), intermediate-term (up to 2 years), and long-term (≥3 years) results were analysed in the meta-analysis. Thirty-day all-cause mortality was lower with EVAR [relative risk (RR) 0.35; 95% CI 0.19–0.64].397 This 66% reduction was consistent in all except for the Anévrisme de l'aorte abdominale: Chirurgie vs. Endoprothèse trial, which quoted similar operative mortality rates for EVAR and open repair (1.3 vs. 0.6%, respectively).394 However, the early benefit in favour of EVAR was gradually lost during follow-up (due to secondary sac ruptures after EVAR), yielding an RR of 0.78 (95% CI 0.57–1.08) at intermediate-term follow-up (≤2 years following procedure) and 0.99 (95% CI 0.85–1.15) at long-term follow-up (>2 years).397 Similarly, the long-term results from the OVER trial suggested a mortality ‘catch-up’ in the EVAR group after 3 years.393 The rate of secondary interventions was considerably higher in the EVAR group at both intermediate (RR 1.48; 95% CI 1.06–2.08) and long-term (RR 2.53; 95% CI 1.58–4.05) follow-up. Similar findings were reported from another meta-analysis that included data from the aforementioned randomized controlled trials and two large registries (Medicare data and Swedish Vascular database).398
Optimal treatment for patients who are unfit for open surgery was addressed only in EVAR-2, a sister trial of EVAR-1. Patients were allocated to either EVAR with best medical care or best medical care alone. The operative mortality of EVAR was 7.3%. Aneurysm-related mortality was significantly lower in the long-term follow-up, but this benefit did not translate into improved all-cause mortality.388 These findings are corroborated by a recently published observational study that included a total of 1652 patients treated by EVAR, of whom 309 (18.7%) were deemed unfit for open repair.399
In conclusion, in patients with suitable anatomy, EVAR is associated with a 66% reduction in operative mortality, a benefit that is lost during follow-up, and which comes at the cost of an increased re-intervention rate. For all other AAA aneurysms that are not suitable for EVAR, open repair remains the reference standard.
7.2.7 (Contained) rupture of abdominal aortic aneurysm
7.2.7.1 Clinical presentation
The classic presentation of ruptured AAA, which includes abdominal pain, hypotension, and abdominal pulsatile mass, may be present in up to 50% of cases. Patients with contained rupture of AAA may present with abdominal or back pain. Since the clinical presentation of ruptured AAA may mimic other abdominal emergencies and early recognition of this condition is imperative, diagnosis cannot be based solely on clinical signs and symptoms and the threshold for immediate imaging should be low.
Recommendations on the management of asymptomatic patients with enlarged aorta or abdominal aortic aneurysm
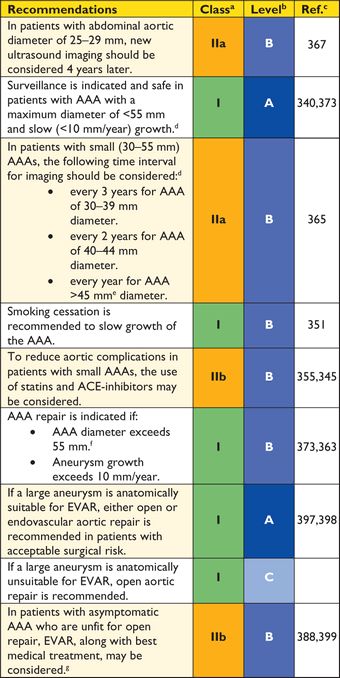 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dWith <1% risk of rupture between two AAA imaging assessments.
eThis interval maybe shortened in women or in the case of rapid growth between previous assessments.
fIndividual decision for operative aneurysm correction should also be influenced by the patient's gender. At a given size, AAAs in women are up to four times as likely to rupture under surveillance, thus aortic repair can be discussed at a lower threshold of probably 50 mm. The patient's life expectancy should also be considered prior to decision for intervention.
gSince only aneurysm-related and not all-cause mortality is improved, informed patient choice is to be taken into account.
AAA = abdominal aortic aneurysm; ACE = angiotensin-converting enzyme; EVAR = endovascular aortic repair.
Recommendations on the management of asymptomatic patients with enlarged aorta or abdominal aortic aneurysm
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dWith <1% risk of rupture between two AAA imaging assessments.
eThis interval maybe shortened in women or in the case of rapid growth between previous assessments.
fIndividual decision for operative aneurysm correction should also be influenced by the patient's gender. At a given size, AAAs in women are up to four times as likely to rupture under surveillance, thus aortic repair can be discussed at a lower threshold of probably 50 mm. The patient's life expectancy should also be considered prior to decision for intervention.
gSince only aneurysm-related and not all-cause mortality is improved, informed patient choice is to be taken into account.
AAA = abdominal aortic aneurysm; ACE = angiotensin-converting enzyme; EVAR = endovascular aortic repair.
7.2.7.2 Diagnostic work-up
In the presence of free, ruptured AAA, massive periaortic bleeding involving the perirenal or pararenal spaces, as well as free fluid in the peritoneal space, allows for a straightforward diagnosis even with ultrasound. Computed tomography is the imaging method of choice in the evaluation of patients with suspected contained- or contained rupture of an AAA. Signs suggesting this condition include a large aneurysm sac, increase of aneurysm size, a thrombus and high-attenuation crescent sign, focal discontinuity in circumferential wall calcification, and the ‘draped aortic sign’.400 This term refers to the combination of an indistinct posterior aortic wall, which lies in close proximity to the adjacent vertebral body, often with loss of the normal fat plane. It may indicate aortic wall insufficiency and contained leak, even in the absence of retroperitoneal bleeding.401
7.2.7.3 Treatment
The preferred treatment strategy for ruptured AAA is currently being investigated in a number of clinical trials.402 The recently published results from the Amsterdam Acute Aneurysm (AJAX) trial showed no significant difference in the combined endpoint of death and severe complication at 30 days, between EVAR and open repair (42 vs. 47%, respectively; absolute risk reduction 5.4%; 95% CI –13–23%).403 Very recent results from the largest study—the Immediate Management of the Patient with Rupture: Open Vs. Endovascular repair trial—yielded similar 30-day mortality results of an endovascular-first strategy and the conventional treatment of immediate repair (35.4 vs. 37.4%, respectively; OR 0.92; 95% CI 0.66–1.28; P = 0.62). All patients with an endovascular-first strategy were sent for immediate CT scan to determine their anatomical suitability for endovascular repair. Suitable patients underwent immediate endovascular repair and the remainder open repair.404
Regarding the patient's gender, for untreated aneurysms the risk of rupture is almost four times as great in women than in men for similar aortic aneurysm diameters. Compared with men, women are exposed to higher periprocedural mortality in elective open and endovascular aneurysm repair.405 The same is true for emergency open repair of ruptured AAA.406 Conversely, a recent systematic analysis did not show a statistically significant increase in risk for mortality in women presenting with ruptured AAA undergoing endovascular repair.407 This is supported by the results from the IMPROVE trial, which suggest that women in particular may benefit from an endovascular strategy.396
7.2.8 Long-term prognosis and follow-up of aortic aneurysm repair
Most patients require a convalescence period of up to 3 months after open AAA repair, after which quality-of-life scores are similar for endovascular and open AAA repair, and even slightly better for open repair at 1 year.408 Open AAA repair is regarded as durable and late, graft-related complications are unusual. Conrad et al. reported a graft-related complication rate of 5.4% at 10 years, while Hallett et al. quoted a rate of 9.4% at an average follow-up of 5.8 years.409,410 The most common complications were anastomotic pseudoaneurysm and graft limb thromboses; graft infection, however, occurs in less than 1%.
Secondary aortic ruptures after open repair are extremely rare; none were reported during long-term follow-up in the EVAR-1 trial.388 Conversely, ruptures after EVAR have been described in many reports and carry a high risk of mortality. These secondary sac ruptures, occurring at a rate of 0.7 per 100 patient-years, were further investigated in the EVAR-1 and EVAR-2 cohorts and were likely to have caused the observed convergence over time, in aneurysm-related mortality, between open repair and EVAR.411 Some specific ‘cluster’ factors, such as Type 1, Type 2, and Type 3 endoleaks, all with sac expansion, kinking, or migration, were associated with late sac ruptures.411
There is some evidence that oral anticoagulation may negatively impact on EVAR outcome due to a higher risk of all types of endoleaks, including persistent Type II, and a loss of endograft sealing. Consequently, close surveillance of EVAR patients on long-term anticoagulation is advised.412,413
Recommendations on management of patients with symptomatic abdominal aortic aneurysm
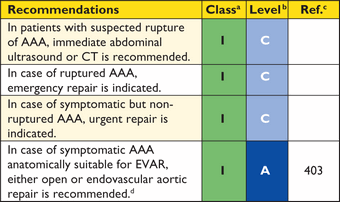 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dDepending on the expertise of the interventional team and patient's level of risk.
AAA = abdominal aortic aneurysm; CT = computed tomography; EVAR = endovascular aortic repair.
Recommendations on management of patients with symptomatic abdominal aortic aneurysm
 |
 |
aClass of recommendation.
bLevel of evidence.
cReference(s) supporting recommendations.
dDepending on the expertise of the interventional team and patient's level of risk.
AAA = abdominal aortic aneurysm; CT = computed tomography; EVAR = endovascular aortic repair.
8 Genetic diseases affecting the aorta
Genetic diseases affecting the aorta are broadly split into two categories: syndromic and non-syndromic, both essentially displaying autosomal dominant transmission. In the past decade, novel underlying gene defects have been discovered in both categories, leading to the constitution of homogeneous molecular groups of thoracic aortic aneurysms and dissection (TAAD). Extensive clinical and imaging studies readily found involvement of the arterial vasculature that was more extensive than just the thoracic aorta. Also, unreported specific alterations were revealed, some shared between the various molecular entities. Finally, large clinical variability is observed within families carrying an identical gene mutation and instances of incomplete penetrance (a ‘skipped generation’) are observed. Both categories and chromosomal or molecular entities of inherited TAAD, as well as non-inherited TAAD, display cystic medial necrosis, thus excluding the use of pathology for making a precise diagnosis.
8.1 Chromosomal and inherited syndromic thoracic aortic aneurysms and dissection
8.1.1 Turner syndrome
Turner syndrome (TS) is essentially caused by partial or complete monosomy of the X chromosome (karyotype 45X0). Diagnosis is based on clinical findings and cytogenetic analyses. Affected women display short stature, various congenital cardiac defects, aortic abnormalities, and metabolic and hormonal alterations leading to obesity, impaired glucose tolerance, hyperlipidaemia, and ovarian failure. Hypertension and brachiofemoral delay are due to coarctation of the aorta, found in 12% of women with TS, usually identified in childhood. Bicuspid aortic valve is found in 30% of patients.414 Approximately 75% of individuals with TS have an abnormal cardiovascular anatomy.415,416 A generalized dilation of major vessels is observed, notably the aorta, the brachial, and carotid arteries. Elongation of the transverse arch and aortic dilation are respectively observed in 30% and 33% of cases, the latter typically located at the root of the ascending aorta. Determination of aortic diameter in adults with TS is, however, difficult in the absence of adequate sex- and age-matched controls of similar body size. The incidence of AD in women with TS is 100 times as great as for women in general, occurring in the third and fourth decades of life.416 The management of adult women with TS associates imaging (echocardiogram and thoracic MRI) with cardiovascular risk assessment. Follow-up will be related to risk categories (absence or number of standard vascular cardiovascular risk factors) with TTE every 3–5 years for low risk, thoracic MRI every 3–5 years for moderate risk, and referral to a cardiologist with 1–2-yearly thoracic MRI for high-risk patients.414 The genetic basis of the disease is still unclear in terms of related cardiovascular and metabolic phenotypes, while short stature has been associated with haploinsufficiency for the SHOX gene.417
8.1.2 Marfan syndrome
Marfan syndrome is the most frequent heritable connective tissue disorder. Transmitted as an autosomal dominant disease, Marfan syndrome is essentially associated with mutations in the FBN1 gene that encodes fibrillin-1, the major component of isolated or elastin-associated microfibrils.418 In a fibrillin-deficient mouse model of Marfan syndrome, enhanced transforming growth factor (TGF)-beta signalling was identified and inhibition of TGF-beta with a neutralizing antibody or with angiotensin-II Type-1 receptor blockers was shown to reverse vascular complications.419 This result was important, since it provided the first new therapeutic option in over 20 years—since the initial report by Shores et al. of the effectiveness of beta-blockade in slowing the rate of aortic dilation, which led to the widespread use of this treatment in Marfan syndrome.98 Several RCTs testing sartans are under way using various Marfan syndrome populations (children and young adults, or adults) and designs (atenolol vs. losartan or losartan vs. placebo on top of optimal therapy).420–422 The results of the two earliest trials (in 20 paediatric/adolescent patients423 and in 233 adults96) show that losartan is effective in reducing the rate of dilation of the aortic root. The results from the other trials are expected in 2014.
Marfan syndrome has already been addressed and recommendations can be found in the Guidelines on the management of grown-up congenital heart disease.424
8.1.3 Ehlers-Danlos syndrome Type IV or vascular type
Ehlers-Danlos syndrome Type IV (EDSIV) is a rare, autosomal, dominant connective tissue disorder caused by mutations in the COL3A1 gene coding for Type III procollagen. Diagnosis is based on clinical signs, non-invasive imaging, and the identification of a mutation in the COL3A1 gene. The clinical features of EDSIV are thin, translucent skin, extensive bruising, characteristic facial appearance (notably a pinched and thin nose, thin lips, prominent ears, hollow cheeks, and tightness of skin over the face), and premature ageing of the skin. Individuals with EDSIV have significantly shortened life spans (50% mortality rate by 48 years) due to the spontaneous rupture of visceral organs (colon, uterus) and blood vessels;425 it affects the entire vascular system and the heart. Fusiform aneurysms are reported. Vascular complications have a tendency to affect arteries of large and medium diameters. The disease frequently involves the thoracic and abdominal aorta, the renal, mesenteric, iliac, and femoral arteries, as well as the vertebral and carotid arteries (extra- and intra-cranial).426 Arteries can dissect without previous dilation and are thus unpredictable. One open randomised trial on 53 affected patients showed a 64% risk reduction of rupture or dissection over 4 years.427 Non-invasive imaging is the preferred approach for evaluating vascular alterations; surgery is only contemplated in potentially fatal complications, since the fragility of tissue, haemorrhagic tendency, and poor wound healing confer an added surgical risk. Prolonged post-operative monitoring is required.428 There are no data to set a threshold diameter for intervention in cases of TAA, and the decision should be based on case by case, multidisciplinary discussion.
8.1.4 Loeys-Dietz syndrome
First described in 2005, Loeys-Dietz syndrome (LDS) is an autosomal dominant aortic aneurysm syndrome combining the triad of arterial tortuosity and aneurysms throughout the arterial tree, hypertelorism, and bifid uvula, as well as features shared with Marfan syndrome.320,429 In some forms, LDS shows a strong overlap with EDSIV. Loeys-Dietz syndrome is associated with mutations in either of the genes encoding the Type I or Type II TGF-beta receptors (TGFBR1 or TGFBR2). Since arterial tortuosity is diagnosed on qualitative observations, a vertebral tortuosity index—measured on a volume-rendered angiogram obtained by thoracic contrast-enhanced MRI—was proposed by Morris et al.430 and was shown to be a reproducible marker of adverse cardiovascular outcomes, not only in LDS but also in other connective tissue disorders where arterial tortuosity is less frequently observed (notably Marfan syndrome and EDS).
Extreme clinical severity is more readily observed in children with prominent craniofacial features (cleft palate, craniosynostosis, retrognathia, exotropia and proptosis) associated with a more severe aortic disease. Observation, in both children and adults, of a widespread and aggressive arteriopathy led to the recommendation of early operative intervention at ascending aortic diameters of ≥42 mm.320 Aggressive surgical management of the aneurysms in patients with LDS is achieved with few complications in the absence of tissue fragility.320,431 However a definite threshold diameter for intervention in cases of TAA cannot be still proposed and the matter requires further investigation. Notably, mutations in the TGFBR2 gene are also found in patients with a Marfan phenotype, who do not display the altered craniofacial features or the widespread and aggressive arteriopathy reported in LDS.432 In contrast to initial studies, which reported dismal clinical outcomes for patients with LDS with TGFBR2 mutations, outcomes appeared similar to those of patients with an FBN1 mutation once the diagnosis was made and medical care given. Conversely, the spontaneous evolution of affected patients who were not medically followed up illustrated the severe prognosis in the absence of care. Patient management is tailored according to extensive vascular imaging at baseline and family history of vascular events.
8.1.5 Arterial tortuosity syndrome
Characterized by arterial tortuosity, elongation, stenosis, and aneurysm of the large- and middle-sized arteries, arterial tortuosity syndrome (ATS) is a very rare autosomal recessive disease. Focal stenoses of the pulmonary arteries and aorta can also be found. Patients display altered facial features (elongated face, blepharophimosis and down-slanting palpebral fissures, a beaked nose, a highly arched palate, and micrognathia) and various signs of a more generalized connective tissue disorder of skin (soft, hyperextensible skin) and skeleton (arachnodactyly, chest deformity, joint laxity, and contractures) overlapping those found in Marfan syndrome. The prognosis was first reported to be poor with mortality rates up to 40% before the age of 5 years.433 A more recent study in families of mostly European origin reported on adult patients, with less-frequent aneurysms and a less-severe vascular phenotype.434 Initially reported in families from Italy, Morocco, and the Middle East, ATS is associated with mutations in the SLC2A10 gene that encodes the facilitative glucose transporter GLUT10.435 Management of patients requires a baseline whole-body vascular imaging, and follow-up should be individually tailored, based on the rate of enlargement of vascular diameters and the family history.
8.1.6 Aneurysms-osteoarthritis syndrome
Aneurysms-osteoarthritis syndrome (AOS) is a new syndromic TAAD that accounts for approximately 2% of familial TAAD.426 This autosomal dominant condition combines early-onset joint abnormalities (including osteoarthritis and osteochondritis dissecans) and aortic aneurysms and dissections. Tortuosity, aneurysms, and dissections are reported throughout the arterial tree.436,437 Mild craniofacial-, skin-, and skeletal features may also be found, overlapping with Marfan syndrome and LDS.437 The disease is associated with mutations in the SMAD3 gene, which encodes an intracellular effector of TGF-beta signalling.438 Diagnosis is based on clinical features and the identification of a mutation in the SMAD3 gene. There is no current consensus on management. Beta-blockade may be beneficial in AOS, since it displays identical aortic alterations to those observed in Marfan syndrome and Loeys-Dietz syndrome, for which this treatment is efficient.436 However, since only limited data are available on the rate of growth of aneurysm, some authors suggest applying the aggressive surgical management recommended for LDS.439
8.1.7 Non-syndromic familial thoracic aortic aneurysms and dissection
Most patients with TAAD do not have a known genetic syndrome. In these patients, familial aggregation with an affected first-degree relative is found in up to 19% of cases. These non-syndromic forms of TAAD (nsTAAD) may be associated with BAV and/or persistent ductus arteriosus,440 and display typical cystic medial necrosis on pathological examination441 Non-syndromic TAAD presents an autosomal dominant transmission with great clinical variability (notably in women) and decreased penetrance.442 Mutations in genes known to be involved in syndromic forms of TAAD (FBN1, TGFBR1, and TGFBR2) are rarely found in families and sporadic patients with nsTAAD.432,443 The effects of mutations in the following new nsTAAD genes have been identified as follows: All of these new molecular entities of nsTAAD and the known gene defects of the syndromic forms now provide a more comprehensive picture of the initiating events of TAAD, with either a connective tissue defect or decreased TGF-beta signalling or altered SMC contractile function. Clinically, these molecular forms display strong overlap and a continuum of gravity of the aortic disease, as well as a more generalized arteriopathy than was previously known. Few data are yet available on the natural history of the new molecular entities of nsTAAD. Diagnosis relies first on exclusion of known genetic syndromes, followed by genetic counselling and investigation of first-degree relatives. Current management strategies combine widespread imaging at baseline and follow-up, according to family history of vascular events.
Mutations in MYH11 (encoding a myosin heavy chain produced in smooth muscle cell [SMC]) associate TAAD and patent ductus arteriosus.444
Mutations in ACTA2 (encoding the SMC-specific alpha-actin) are found in patients with TAAD also presenting with coronary artery disease, stroke, and Moyamoya disease.445
Mutations in MYLK (encoding myosin light chain kinase) lead to AD with little to no aortic enlargement.446
Mutations in TGFB2 (encoding TGF-beta Type 2) result in TAAD with some overlap with Marfan syndrome for skin and skeletal features.446
Mutations in PRKG1 (encoding PKG I, a Type I cGMP-dependent protein kinase that controls SMC relaxation) result in aortic aneurysm and acute ADs at relatively young ages.447
Recommendations on genetic testing in aortic diseases
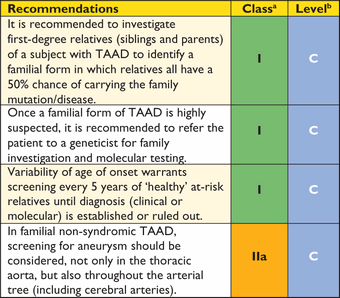 |
 |
aClass of recommendation.
bLevel of evidence.
TAAD = thoracic aortic aneurysms and dissection.
Recommendations on genetic testing in aortic diseases
 |
 |
aClass of recommendation.
bLevel of evidence.
TAAD = thoracic aortic aneurysms and dissection.
8.1.8 Genetics and heritability of abdominal aortic aneurysm
Since the first report of three brothers with AAA by Clifton in 1977,447 many studies have reported familial aggregation of AAA among siblings of patients with that condition.448 There is a 24% probability that a monozygotic twin of a person with an AAA will develop an aneurysm.449 However, the proportion of patients with AAA who have first-degree relatives with the disease is usually low in cohort studies, although it does vary between 1% and 29%.450
In the minority of families with multiple AAA cases, segregation analyses have been performed and have led to models of either autosomal recessive- or autosomal dominant inheritance.451,452 Despite reports of these rare families, the development of AAAs is generally unlikely to be related to a single gene mutation and multiple genetic factors are implicated. Thus susceptibility genes, rather than causal gene mutations, are likely to be important, particularly those regulating inflammatory mediators, tissue proteases, and smooth muscle cell (SMC) biology. A note of caution should be added in view of the recent description of familial forms of TAA in which AAAs are observed. Therefore, if AAA occurs in a young subject with no overt risk factors and without other affected family members to investigate, then a more widespread arterial disease should be screened, notably in the thoracic aorta.
8.2 Aortic diseases associated with bicuspid aortic valve
Valvular problems associated with BAV are covered in the 2012 ESC Guidelines on the management of valvular heart disease.312
8.2.1 Epidemiology
8.2.1.1 Bicuspid aortic valve
BAV is the most common congenital cardiac defect, with a prevalence at birth of 1–2%. Males are more often affected than females, with the ratio ranging from 2:1 to 4:1.453–456 BAV is the result of fusion of the left coronary cusp (LCC) and right coronary cusp (RCC) in >70% of patients, of fusion of the RCC with the non-coronary cusp (NCC) in 10–20%, and due to fusion of LCC with NCC in 5–10%.457 True bicuspid valves and unicommisural valves are very rare.
8.2.1.2 Ascending aorta growth in bicuspid valves
Aortic dilation, defined as an aorta diameter of >40 mm irrespective of body surface area,458–460 or of >27.5 mm/m2 for people of short stature, is frequently associated with BAV. The risk of development of aortic dilation in patients with BAV is probably much higher than in the normal population, 313 but there are no reliable population-based data on its incidence. There are some indications on racial differences in the extent of aortic dilation in BAV.461
Various subtypes of BAV are associated with different forms of aortic dilation.462 In patients with an LCC–RCC type BAV, ascending aorta dilation is common, but aortic root dilation is also seen.463 In the RCC–NCC type, the aortic root is rarely affected and only dilation of the ascending aorta is seen.313 Aortic dilation is maximal at the level of the tubular aorta, with a mean rate of 0.5 mm/year, similar to that seen in Marfan patients.316 However, in this population, 50% of the patients do not present aortic dilation over a 3-year period, whereas other do,316 emphasizing the heterogeneity of the population of patients with BAV. The aortic arch is rarely affected.464 Data to quantify the strength of these associations are not available.
Beyond aortic dilation and aneurysm formation, BAV is a risk factor for dissection and rupture.465 Patients with BAV, including those with a haemodynamically normal valve, have dilated aortic roots and ascending aortas, compared with age- and sex-matched control subjects.466 Among adults with BAV and no significant valve disease at baseline, 27% will require cardiovascular surgery within 20 years.467 The mean growth rate of proximal ascending aortic aneurysms in patients with BAV and aortic stenosis is greater than that seen in patients with tricuspid valves (1.9 vs. 1.3 mm/year, respectively).465 In another study in patients with a normally functioning BAV, an annual growth rate of 0.77 mm was reported.468 Average annual changes in the ascending aorta in patients with BAV may vary from 0.2 to 1.2 mm/year.316,466,469 Aortic dilation rate is higher in the tubular ascending aorta than in the sinuses of Valsalva, which differs from Marfan syndrome.316 In patients with BAV who had untreated aortic dilation at the time of aortic valve replacement, the 15-year rate of aortic surgery or complications was reported to be as high as 86% when the initial aortic diameter was <40 mm, 81% with diameters from 40–44 mm, and only 43% for diameters from 45–49 mm, respectively (P < 0.001).470 Another study found a low risk of adverse aortic events after isolated valve replacement in patients with BAV stenosis and concomitant mild-to-moderate dilation of the ascending aorta (40–50 mm) with only 3% of patients requiring proximal aortic surgery at up to 15 years follow-up.471
8.2.1.3 Aortic dissection
One study reported a cumulative incidence of 6% of Type A AD in untreated patients with BAV and aortic dilation over a mean follow-up of 65 months,465 but in the current era of early preventive surgery this is difficult to assess. There are no reliable historical data. The prevalence of BAV ranges from 2–9% in Type A AD and 3% in Type B AD,472 both only slightly higher than the prevalence of BAV in the general population (1–2%).
8.2.1.4 Bicuspid aortic valve and coarctation
Only the LCC–RCC type of BAV is associated with aortic coarctation.473,474 Data on the prevalence of aortic coarctation in BAV are scarce: one report states 7%.313 In contrast, among patients with a coarctation, 50–75% have a BAV (of the LCC–RCC type). In patients with coarctation and BAV, the risk of developing aortic dilation and dissection is much higher than in the population with BAV only.475,476
8.2.2 Natural history
Reports on the enlargement of aortic dimensions vary. Mean progression is reported to be 1–2 mm/year,65,469 but faster growth occurs occasionally. Rapid progression of >5 mm/year and larger diameters are associated with increased risk of AD or rupture, with a sharp increase of risk at a diameter >60 mm. A higher gradient across a stenotic BAV and more severe aortic regurgitation (higher stroke volume) are reported to be associated with faster increase in aortic dimensions.477 In the absence of stenosis or regurgitation, severe dilation also can occur, especially in young adults.478,479
Data on the increase in aortic dimensions after valve replacement show that re-operation for an aortic root with a diameter of 40–50 mm during the valve replacement is rarely necessary after a follow-up of >10 years. Dissection is very rare in this group.471,480
8.2.3 Pathophysiology
Notch1 gene mutations are associated with BAV.481 A high incidence of familial clustering was observed, compatible with autosomal dominant inheritance with reduced penetrance.
Different orientations of the leaflets (fusion of LCC to RCC or RCC to NCC) seem to have distinct aetiologies in the embryonic phase.482 Different types of BAV are associated with different forms of aortic pathology but the pathophysiology behind this remains unknown.313 It might be either genetic, with common genetic pathways for aortic dilation and BAV,483,484 or consecutive to altered aortic flow patterns in BAV,485–487 or a combination of both.
8.2.4 Diagnosis
8.2.4.1 Clinical presentation
BAV, with stenosis or regurgitation, can give rise to complaints and clinical signs (heart murmurs) that can be detected on clinical examination. A dilating aorta is rarely symptomatic. Chronic chest, neck, and back pain can be atypical signs of a dilated aorta. Dyspnoea, inspiratory stridor, and recurrent airway infection may indicate compression of major airways. Hoarseness may indicate compression of the laryngeal nerve. The first clinical manifestation of untreated progressive aortic dilation associated with BAV is often aortic rupture or AD. A small subset of patients with BAV (<15%), almost exclusively young men, presents predominantly with aortic root dilation without substantial valvular stenosis or regurgitation, with very few or no clinical symptoms. These patients are at risk, but are very difficult to identify if not detected by means of screening.
8.2.4.2 Imaging
There are no specific comments regarding imaging of the aorta in this setting.
8.2.4.3 Screening in relatives
Because of BAV's strong familial association,453,483,488 screening of first-degree relatives may be considered. There are no data about the effectiveness (i.e. number of patients to screen to diagnose one otherwise undetected patient) or cost-effectiveness of a screening programme.
8.2.4.4 Follow-up
In every newly diagnosed patient with BAV, the aortic root and ascending aorta should be visualized with TTE alone or associated with another imaging modality, preferably MRI. If TTE is feasible, there is a good correlation between MRI and TTE and, when the aorta is not dilated, annual follow-up can be done with TTE, with intervals depending on rate of enlargement and/or family history. In cases of an increase in diameter >3 mm/year or a diameter >45 mm measured on TTE, a measurement with another imaging modality (MRI or CT) is indicated. From a diameter of 45 mm, annual follow-up of the ascending aorta is advised. If TTE cannot reliably visualize the ascending aorta, annual imaging with MRI (or CT if MRI is not possible) is indicated.489
8.2.5 Treatment
Although there are no studies that provide evidence that medical treatment of a dilated aorta has any effect on the enlargement of the ascending aorta or aortic root in BAV, it is common clinical practice to advise beta-blocker therapy when the aorta is dilated. The indication for surgical treatment of aortic dilation in BAV is similar to that for other causes of dilation, except for Marfan syndrome. When surgery is indicated for BAV, stenosis or regurgitation, aortic root replacement should be considered if the root is larger than 45 mm in diameter,470 because of elevated risk of aortic dilation necessitating intervention (or dissection or rupture) in the years following surgery.
8.2.6 Prognosis
The risk of dissection and rupture increases with the diameter of the aorta, with a sharp increase at a diameter of 60 mm. When treated according to guidelines, the prognosis is favourable—much better than that of Marfan syndrome–and similar to that of an age-matched normal population.313,485
Recommendations for the management of aortic root dilation in patients with bicuspid aortic valve
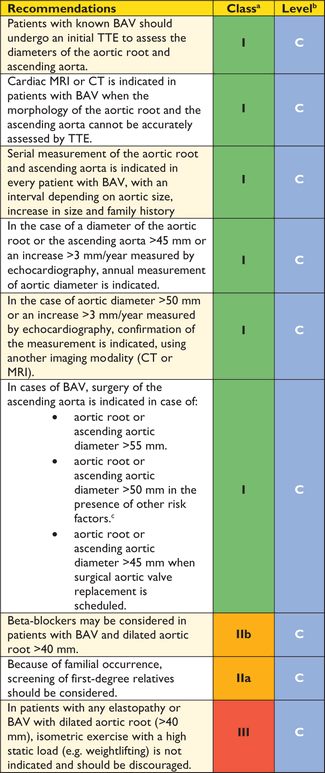 |
 |
aClass of recommendation.
bLevel of evidence.
cCoarctation of the aorta, systemic hypertension, family history of dissection, or increase in aortic diameter >3 mm/year (on repeated measurements using the same imaging technique, measured at the same aortic level, with side-by-side comparison and confirmed by another technique).
BAV = bicuspid aortic valve; CT = computed tomography; MRI = magnetic resonance imaging; TTE = transthoracic echocardiography.
Recommendations for the management of aortic root dilation in patients with bicuspid aortic valve
 |
 |
aClass of recommendation.
bLevel of evidence.
cCoarctation of the aorta, systemic hypertension, family history of dissection, or increase in aortic diameter >3 mm/year (on repeated measurements using the same imaging technique, measured at the same aortic level, with side-by-side comparison and confirmed by another technique).
BAV = bicuspid aortic valve; CT = computed tomography; MRI = magnetic resonance imaging; TTE = transthoracic echocardiography.
8.3 Coarctation of the aorta
This topic is discussed extensively in the 2010 ESC Guidelines on the management of grown-up congenital heart disease.424
8.3.1 Background
Coarctation of the aorta is considered to be a complex disease of the vasculature and not only as a circumscript narrowing of the aorta. It occurs as a discrete stenosis or as a long, hypoplastic aortic segment. Coarctation of the aorta is typically located at the area of ductus arteriosus insertion, and occurs ectopically (ascending, descending, or abdominal aorta) in rare cases. Coarctation of the aorta accounts for 5–8% of all congenital heart defects. The prevalence of isolated forms is 3 per 10 000 live births.
8.3.2 Diagnostic work-up
Clinical features include upper body systolic hypertension, lower body hypotension, a blood pressure gradient between the upper and lower extremities (>20 mm Hg indicates significant coarctation of the aorta), radiofemoral pulse delay, and palpable collaterals. Echocardiography provides information regarding site, structure, and extent of coarctation of the aorta, left ventricular function and hypertrophy, associated cardiac abnormalities, and aortic and supra-aortic vessel diameters. Doppler gradients are not useful for quantification, neither in native nor in post-operative coarctation. MRI and CT are the preferred non-invasive techniques to evaluate the entire aorta in adults. Both depict site, extent, and degree of the aortic narrowing, the aortic arch, the pre- and post-stenotic aorta, and collaterals. Both methods detect complications such as aneurysms, re-stenosis, or residual stenosis. Cardiac catheterization with manometry (a peak-to-peak gradient >20 mm Hg indicates a haemodynamically significant coarctation of the aorta in the absence of well-developed collaterals), and angiography are still the ‘gold standard’ for evaluation of this condition at many centres before and after operative or interventional treatment.
8.3.3 Surgical or catheter interventional treatment
In native coarctation of the aorta with appropriate anatomy, stenting has become the treatment of first choice in adults in many centres.
Recommendations on interventions in coarctation of the aorta
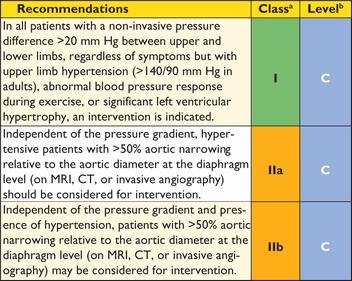 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; MRI = magnetic resonance imaging.
Recommendations on interventions in coarctation of the aorta
 |
 |
aClass of recommendation.
bLevel of evidence.
CT = computed tomography; MRI = magnetic resonance imaging.
The question of whether to use covered or non-covered stents remains unresolved. Notably, despite intervention, antihypertensive drugs may still be necessary to control hypertension.
9. Atherosclerotic lesions of the aorta
9.1 Thromboembolic aortic disease
As a result of the atherosclerotic process, aortic plaques consist of the accumulation of lipids in the intima-media layer of the aorta.490 Secondary inflammation, fibrous tissue deposition, and surface erosions with subsequent appearance of thrombus may cause either thrombotic (thromboembolic) or atherosclerotic (cholesterol crystal) embolism.491
Thromboemboli are usually large, and commonly occlude medium-to-large arteries, causing stroke, transient ischaemic attack, renal infarct, and peripheral thromboembolism. Cholesterol crystal emboli tend to occlude small arteries and arterioles, and may cause the ‘blue-toe’ syndrome, new or worsening renal insufficiency, and mesenteric ischaemia.
9.1.1 Epidemiology
Risk factors are similar to those for atherosclerosis in other vascular beds, including age, sex, hypertension, diabetes mellitus, hypercholesterolaemia, sedentary lifestyle, tobacco smoking, and inflammation. In the Offspring Framingham Heart Study, aortic plaque was identified by MRI in 46% of normotensive individuals, with a greater prevalence in women. Hypertension was associated with greater aortic plaque burden. An even greater plaque burden was present in subjects with clinical cardiovascular disease.492
Aortic plaques are associated with cerebrovascular and peripheral embolic events. The association between cerebrovascular and embolic events is derived from autopsy studies,493 and studies in patients with non-fatal cerebrovascular or peripheral vascular events,494 as well as those in high-risk patients referred for TOE and intraoperative ultrasound.495,496 In the Stroke Prevention in Atrial Fibrillation study, patients with complex aortic plaque (defined by plaques with mobile thrombi or ulcerations or a thickness ≥4 mm by TOE) had a risk of stroke four times as great compared with plaque-free patients.497 In The French Study of Aortic Plaques in Stroke,498 aortic plaques ≥4 mm were independent predictors of recurrent brain infarction (RR = 3.8) and any vascular events (RR = 3.5). The prevalence of severe aortic arch atheroma among patients with acute ischaemic stroke is >20%, similar to atrial fibrillation and carotid atherosclerosis.499 Additionally, most of the studies noted that progression of atheroma was associated with more vascular events.500
Embolic events can also be induced by interventions including cardiac catheterization, intra-aortic balloon counter-pulsation, and cardiac surgery. For cardiac catheterization, the overall risk of stroke is low. In a recent meta-analysis, stroke rates tended to be lower with the radial vs. femoral approach without reaching statistical significance (0.1 vs. 0.5%, respectively; P = 0.22).501 Atherosclerosis of the ascending aorta is a major risk factor for stroke after cardiac surgery. The level of risk depends on the presence, location, and extent of disease when the ascending aorta is surgically manipulated. In a study of 921 patients undergoing cardiac surgery, the incidences of stroke in patients with and without atherosclerotic disease of the ascending aorta were 8.7% and 1.8%, respectively (P < 0.0001).502 Intraoperative (epiaortic ultrasonography) or pre-operative diagnosis and surgical techniques such as intra-aortic filters, off-pump coronary artery bypass, single aortic clamp or no clamping, and ‘no-touch’ off-pump coronary artery bypass may prevent embolic events.503 Nowadays, transcatheter aortic valve implantation is mostly proposed in the elderly with multiple comorbidities, and these patients are at high risk for aortic plaques, which are in part responsible for procedure-related stroke, as highlighted by lower stroke rates when the aortic catheterization is avoided by the transapical approach.504
9.1.2 Diagnosis
Aortic atheroma can be subdivided in small, moderate, and severe aortic atherosclerosis, or even semi-quantitatively into four grades (Web Table 3).505,506
TTE offers good imaging of the aortic root and proximal ascending aorta. TOE is a safe and reproducible method of assessing aortic atheromas.507 Multiplanar real-time 3D TOE may offer further advantages. Epiaortic ultrasonography (2D or 3D)508 can offer valuable data during the intraoperative setting. Multislice computed tomography can offer excellent imaging of aortic atheromas and gives valuable data on anatomy and calcifications. Magnetic resonance imaging can give details on the composition of plaques. The limitations of each technique are detailed in section 4.
9.1.3 Therapy
9.1.3.1 Antithrombotics (antiplatelets vs. vitamin K antagonists)
Because of the thromboembolic risk, antiplatelet therapy or anticoagulation is considered.498 However, studies comparing both options are scarce and mostly small and non-randomized.482 Warfarin has been used for primary or secondary prophylaxis in patients with aortic plaque. In an observational study including 129 patients,509 a lower incidence of vascular and fatal events was found in the case of complex plaques in patients on vitamin K antagonist vs. antiplatelet therapy (aspirin or ticlopidine). Other studies also reported beneficial results.510,511 Nevertheless, other groups reported no benefit with warfarin use: in a study of 519 patients with severe aortic plaque the OR for embolic events was 0.7 (95% CI 0.4–1.2) for warfarin and 1.4 (95% CI 0.8–2.4) for antiplatelet agents.512 In the Patent Foramen Ovale in Cryptogenic Stroke study (PICSS), based on the Warfarin-Aspirin Recurrent Stroke Study (WARSS),513 event rates for the entire population (n = 516, of whom 337 had aortic plaques) were similar in the warfarin and aspirin groups (16.4 vs. 15.8%; P = 0.43) and no correlation was observed between warfarin treatment and large plaques on the risk of events (HR 0.42; 95% CI 0.12–1.47).
More data are needed to allow for better selection of patients and to determine firm recommendations. The promising Aortic Arch Related Cerebral Hazard (ARCH) trial, comparing warfarin (target international normalized ratio 2–3) with aspirin plus clopidogrel, has been prematurely stopped because of a lack of power for a definite result. In the Stroke Prevention in Atrial Fibrillation III study514 the co-existence of aortic plaque in patients with atrial fibrillation dramatically increased the risk of embolic events. Aortic plaque is considered as ‘vascular disease’ and increases, by one point, the CHA2DS2-VASc score used to assess the stroke risk in atrial fibrillation.515
9.1.3.2 Lipid-lowering agents
No randomized trials are available to support the use of statins for patients with stroke caused by atheroembolism. In a small series of patients with familial hypercholesterolaemia who were studied with TOE, pravastatin resulted in progression in 19% and regression in 38% over 2 years.516 Statin use results in regression of aortic atheroma burden as assessed by MRI,517 or attenuation of inflammation as assessed by PET.518 More research is required to clarify the value of statins and the risk of stroke in patients with large aortic plaques. In a retrospective study of 519 patients with severe aortic plaque, only statin treatment was associated with a 70% lower risk of events.512
9.1.3.3 Surgical and interventional approach
There are limited data—mainly from case studies—and no clear evidence to recommend prophylactic endarterectomy or aortic arch stenting for prevention of stroke. Surgery for atherothrombotic disease in the aortic arch is of a high-risk nature and cannot be recommended.519
Recommendations on management of aortic plaque
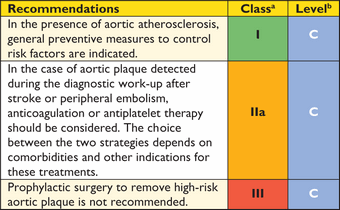 |
 |
aClass of recommendation.
bLevel of evidence.
Recommendations on management of aortic plaque
 |
 |
aClass of recommendation.
bLevel of evidence.
9.2 Mobile aortic thrombosis
Mobile thrombi in the aorta of young patients without diffuse atherosclerosis have been reported since the regular use of TOE in patients with cerebral or peripheral emboli, mostly located at the aortic arch. The pathophysiology of these lesions is unclear, since thrombophilic states are not frequently found.520 In the largest series of 23 patients (of 27 855 examinations) with mobile thrombi of the aortic arch, only four cases presented thrombophilic states. Thrombi may present a paradoxical embolism via an open foramen ovale. The thrombi were attached either on a small aortic plaque or a visually normal wall. Medical treatment (heparinization), endovascular stenting, or surgery have been proposed, but no comparative data are available.
9.3 Atherosclerotic aortic occlusion
Abdominal aortic occlusion is rare and results in a major threat of leg amputation or death. Extensive collateralization usually prevents the manifestation of acute ischaemic phenomena.520 Aortic occlusion can also be precipitated by hypercoagulable states. Aetiopathogenic factors of the disease include small vessel size, cardiac thromboembolism, AD, and distal aortic coarctation. This condition may be either asymptomatic or present with sudden onset of intermittent claudication. Symptoms may worsen progressively until low flow leads to obstruction of collateral vasculature, causing severe ischaemic manifestation in the lower extremities, the spinal cord, intestine and kidney, depending on the site and extension of obstruction. The diagnosis is mostly made with the use of Doppler ultrasonography. Other imaging techniques (CT or MRI) yield more detailed information that can guide the planning of treatment. Treatment may be bypass grafting or aorto-iliac endarterectomy. Endovascular therapy has also been proposed.
9.4 Calcified aorta
Calcification occurs in the media, and the amount of calcification is directly associated with the extent of atherosclerosis. The presence of severe atherosclerosis of the aorta causes an eggshell-like appearance visualized on chest X-ray (porcelain aorta). The calcification interferes significantly with cannulation of the aorta, cross-clamping, and placement of coronary bypass grafts, significantly increasing the risk of stroke and distal embolism. Off-pump coronary bypass and the implantation of transcatheter aortic heart valves may render a solution in patients requiring, respectively, coronary bypass grafting and aortic valve replacement with porcelain aorta [15.1% of patients in the Placement of AoRtic TraNscathetER Valves (PARTNER) cohort B trial with aortic stenosis were inoperable due to porcelain aorta].521
9.5 Coral reef aorta
‘Coral reef’ aorta is a very rare calcifying stenotic disease of the juxta renal and suprarenal aorta. Only case reports exist, except for one group reporting a series of >80 cases, most of them women, over 24 years.522 Coral reef aorta is described as rock-hard calcifications in the visceral part of the aorta. These heavily calcified plaques grow into the lumen and can cause significant stenosis, which may develop into bowel ischaemia, renal failure, or hypertension due to renal ischaemia. The aetiology and pathogenesis are still uncertain although it has been proposed that calcification of a fibrin-platelet thrombus may result in this lesion. This may occur at the site of an initial injury to the aortic endothelium. Vascular surgery was used in the past but, recently, endovascular interventions play a greater role, particularly in high-risk individuals with multiple comorbidities.523
10. Aortitis
10.1 Definition, types, and diagnosis
Aortitis is the general term used to define inflammation of the aortic wall. The most common causes of aortitis are non-infectious inflammatory vasculitis, namely giant cell (or temporal) arteritis (GCA) and Takayasu arteritis (Web Table 4).524,525 Non-infectious aortitis has also been described in other inflammatory conditions such as Becet's disease,526 Buerger disease, Kawasaki disease, ankylosing spondylarthritis, and Reiter's syndrome.527 Although less common, infections due to Staphylococcus, Salmonella, and mycobacteria have been reported to cause infective aortic disease, supplanting the infection by Treponema pallidum in the past.528
10.1.1 Giant cell arteritis
Giant cell arteritis tends to affect the older population, more often by far in women than in men. When the aorta is affected, it may result in thoracic aortic aneurysm. Although, classically, the temporal and/or other cranial arteries are involved, the aorta and its major branches are affected in approximately 10–18% of cases.514,524,528 Dilations of the aortic root and ascending aorta are common and can lead to AD or rupture.524 If a diagnosis of extracranial GCA is suspected, echocardiography, CT, or MRI are recommended.529 A thickened aortic wall on CT or MRI indicates inflammation of the aortic wall, and thus active disease.530 Studies with PET scanning have suggested that subclinical aortic inflammation is often present in patients with GCA.531 Along with the usual inflammatory markers, measurement of interleukin-6 may be useful in patients with suspected GCA.
10.1.2 Takayasu arteritis
Takayasu arteritis is a rare, large-vessel vasculitis of unknown aetiology, typically affecting young women.532 It occurs most often in the Asian population. The overall rate is 2.6 per million inhabitants.533 The thoracic aorta and its major branches are the most frequent locations of the disease, followed by the abdominal aorta. While the initial stages of the disease include signs and symptoms of systemic inflammation, the chronic phase reflects vascular involvement. The clinical presentation of Takayasu arteritis varies across a spectrum of symptoms and clinical signs, ranging from back- or abdominal pain with fever to acute severe aortic insufficiency, or to an incidentally identified large thoracic aortic aneurysm.525,528,532
Upper extremity claudication, stroke, dizziness, or syncope usually indicate supra-aortic vessel obstruction. Hypertension is sometimes malignant and suggests narrowing of the aorta or renal arteries. AAS, including AD and rupture, can occur. Inflammation-associated thrombus formation in the aortic lumen with peripheral embolization has also been reported.528,532
In the case of suspicion of Takayasu arteritis, imaging the entire aorta is of critical importance, to establish the diagnosis. All imaging modalities play an important role in the diagnosis and follow-up of Takayasu arteritis. Digital subtraction angiography of the aorta and its branches provides only information regarding luminal changes, a late feature in the disease course.530 Echocardiography, MRI, and CT are useful in demonstrating homogeneous circumferential thickening of the aortic wall with a uniform smooth internal surface.529 This finding could be misdiagnosed as an IMH. Compared with echography, CT and MRI provide better assessment of the entire aorta and its proximal branches, as well as distal pulmonary arteries that are sometimes affected. MRI may show arterial wall oedema, a marker of active disease.528,530 In the chronic stage, the aortic wall may become calcified, best assessed by CT. A PET scan may be particularly useful in detecting vascular inflammation when combined with traditional cross-sectional imaging modalities.531 Inflammation biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in approximately 70% of patients in acute phase and 50% in the chronic phase of the disease.528 Pentraxin-3 may have a better accuracy in differentiating the active- from the inactive phase of Takayasu arteritis.
10.2 Treatment
In non-infectious aortitis, corticosteroids are the standard initial therapy.534 In general, an initial dose of 0.5–1 mg/kg prednisone daily is prescribed. This treatment is typically required for 1–2 years to avoid recurrence, although the dose may be tapered off 2–3 months after initiation. Despite this prolonged regimen, nearly half of patients will relapse during tapering, requiring additional immunosuppression.535 In addition to recurrent symptoms, re-elevation of inflammatory markers may be a helpful sign of relapse, particularly among patients with GCA. The value of oedema-weighted MRI and 18F-FDG PET in the diagnosis of relapse in Takayasu arteritis is an area under continuing investigation. Second-line agents include methotrexate, azathioprine, and anti-tumour necrosis factor-alpha agents.536
A comprehensive vascular examination should be performed at each visit, in combination with follow-up of inflammation biomarkers and periodic imaging for the development of thoracic or abdominal aortic aneurysm, given the known risk of these complications.524,528 The indications for revascularization for aortic stenosis or aneurysm are similar to those in non-inflammatory disorders. The risk of graft failure is higher in patients with active local inflammation.537–539 Ideally, patients should be in clinical remission before elective repair of an aortitis-related aneurysm.528,534
Suspected infectious aortitis requires rapid diagnosis and intravenous antibiotics with broad antimicrobial coverage of the most likely pathological organisms (particularly Staphylococcal and gram-negative species).
11. Aortic tumours
11.1 Primary malignant tumours of the aorta
Primary malignant tumours of the aorta are an extremely rare class of sarcomas exhibiting a wide histopathological heterogeneity. Intimal sarcomas, the most common, are derived from endothelial cells (angiosarcoma) or from myofibroblasts. Leiosarcomas and fibrosarcomas originate from the media or adventitia of the aortic wall.541
The symptoms associated with aortic tumours are non-specific and mimic atherosclerotic disease of the aorta, peripheral artery diseases, gastrointestinal or renal pain syndromes, or vertebral disk herniation. The most characteristic and frequently reported clinical presentation of an intimal angiosarcoma of the aorta is the embolic occlusion of the mesenteric or peripheral artery. Most often the ante mortem diagnosis is made by immunohistopathological examination of endarterectomy or aortic resection specimens. Only in a very small number of cases the diagnosis is suspected on pre-operative MRI of the aorta.
Owing to its atypical and highly variable symptomatology, this very rare condition is most often diagnosed only in an advanced stage. In patients with peripheral or splanchnic emboli, an aortic sarcoma should be included in the differential diagnosis, especially in patients with mild or absent underlying atherosclerotic disease. After a cardiac source of the embolism is ruled out, contrast-enhanced MRI of the thoracic and abdominal aorta should be performed, as this investigation is the most sensitive diagnostic tool for detection of an aortic tumour. If an aortic lesion is found that is suggestive of a sarcoma, additional ultrasound examination may demonstrate inhomogeneity of the lesion, which is atypical for a mural thrombus. If the diagnosis of an aortic sarcoma is suspected, bone scintigraphy is recommended owing to the high prevalence of bone metastasis.
Based on reported cases, the recommended therapy involves en bloc resection of the tumour-involved portion of the aorta with negative surgical margins, followed by graft interposition; however, owing to the late diagnosis—frequently at a stage already complicated by the presence of metastases, the location of the aortic lesion, or the presence of comorbidities—this intervention is mostly unfeasible. Other approaches can be endarterectomy or endovascular grafting of the involved segment of the aorta. Adjuvant or palliative chemotherapy and radiation have been used in selected cases and may result in a prolonged survival.
The prognosis for aortic sarcomas is poor, with metastatic disease leading to death in a short time in most patients. Mean survival from the time of diagnosis is 16 ± 2.4 months.541 Overall survival at 3 years is 11.2%. Following surgical resection, the 3-year survival rates increased to 16.5%.542
12. Long-term follow-up of aortic diseases
Patients with aortic disease usually require life-long surveillance, regardless of the initial treatment strategy (medical, interventional, or surgical). This surveillance consists of clinical evaluation, reassessment of a patient's medical therapies and treatment goals, as well as imaging of the aorta. This section includes the chronic phase of AD after discharge and as well as specific aspects of follow-up in patients who took benefit from an aortic intervention.
12.1 Chronic aortic dissection
12.1.1 Definition and classification
Survivors of an acute AD ultimately enter a chronic disease course. Previously, AD was considered chronic 14 days after onset of symptoms. It is now accepted practice to further divide the time course of AD into acute (<14 days), sub-acute (15–90 days), and chronic (>90 days) phases. Chronic AD can either be uncomplicated, with a stable disease course, or complicated by progressive aneurysmal degeneration, chronic visceral or limb malperfusion, and persisting or recurrent pain or even rupture. Patients with chronic AD also include those previously operated for Type A AD, with persisting dissection of the descending aorta.
12.1.2 Presentation
Two clinical patterns should be distinguished: patients with initially acute AD entering the chronic phase of the disease and those in whom first diagnosis of chronic AD is made. Patients with newly diagnosed chronic AD are often asymptomatic. The lesion is found incidentally as mediastinal widening or prominent aortic knob on chest X-ray. In these patients, the exact timing of dissection is often difficult. The patient's history has to be carefully evaluated for a previous acute pain event. Infrequently, patients may also present symptoms related to the enlarging dissected aorta (hoarseness, new onset chest pain), or chronic malperfusion (abdominal pain, claudication, altered renal function) or acute chest pain indicating rupture.
12.1.3 Diagnosis
Diagnosis has to be confirmed by cross-sectional imaging such as contrast-enhanced CT, TOE, or MRI. Chronicity of AD is suggested by imaging characteristics: thickened, immobile intimal flap, presence of thrombus in the FL, or aneurysms of the thoracic aorta secondary to chronic AD, mostly developed in the distal aortic arch. In symptomatic patients, signs of (contained) rupture such as mediastinal haematoma or pleural effusion may be present.
12.1.4 Treatment
In patients with chronic, uncomplicated Type B AD, a primary approach with medical therapy and repetitive clinical and imaging follow-up is recommended. Competitive sports and isometric heavy weight lifting should be discouraged, to reduce aortic wall shear stress due to sudden rises in arterial blood pressure during such exercise. Body contact sport activities should also be discouraged, while leisure sportive activities with low static/low dynamic stress are acceptable.
Blood pressure should be lowered to <130/80 mm Hg. Weight lifting activities should be restricted to avoid blood pressure peaks. Beta-blockers have been seen to be associated with reduced aneurysmal degeneration of the dissected aorta and reduced incidence of late dissection-related aortic procedures in non-randomized studies.543 A contemporary analysis of the IRAD database, comprising a total of 1301 patients with Type A and Type B acute AD, showed that beta-blockers (prescribed to 88.6% of patients) were the most commonly used medication and suggested that their use was associated with improved survival.544 Calcium channel blockers were associated with improved survival, selectively in those with Type B dissections, while renin-angiotensin system inhibitors were not significantly associated with survival.544 Angiotensin-1 antagonists (losartan) are conceptually attractive and have been shown to slow aortic enlargement in Marfan patients.96,545 No data exist on the use of angiotensin-1 blockers in chronic AD. So far, angiotensin-1 blockers may be considered for antihypertensive combination therapy if beta-blockers alone do not achieve the blood pressure target.
The INvestigation of STEnt-grafts in Aortic Dissection trial did not show any survival benefit of TEVAR over optimal medical therapy in patients with asymptomatic sub-acute/chronic AD during 2-year follow-up.218,219 The 5-year aorta-related mortality was 0% vs 16.9%, respectively, in TEVAR plus medical therapy vs. medical therapy alone. All-cause mortality at 5 years was 11.1% vs. 19.3%, respectively (P = not significant), and progression 27% vs. 46.1% (P = 0.04). Morphological results were, however, significantly improved by TEVAR (aortic remodelling 91.3% with TEVAR vs. 19.4%). It should be noted that 16% of patients initially randomized to optimal medical therapy required crossover to TEVAR due to evolving complications during follow-up. Deferred TEVAR could be successfully performed in these patients without increased mortality or complications. A recent multicentre study from China, covering 303 patients with chronic AD, showed lower aorta-related mortality for TEVAR than with medical therapy but failed to improve the overall survival rate or lower the rate of aorta-related adverse events.546
Patients with chronic Type B AD that is complicated by progressive thoracic aortic enlargement (>10 mm/year), FL aneurysms (with total aortic diameter >60 mm), malperfusion syndrome, or recurrent pain, require TEVAR or surgical treatment. The optimal treatment in patients with chronic AD is, however, unclear. No randomized comparison of TEVAR and conventional surgery exists. Thoracic endovascular aortic repair may be used to exclude the aneurysm, which is typically located in the distal aortic arch, and prevent rupture; however, aortic remodelling cannot be expected, due to the thickened, immobile intimal flap. Smaller case series have shown that TEVAR is feasible in patients with aneurysm of the descending thoracic aorta secondary to chronic AD, with an acceptable mid-term outcome.547 Complete aortic remodelling was observed in only 36% of patients after TEVAR.547 In a review of 17 studies including 567 patients,548 the technical success rate was 89.9%, with mid-term mortality 9.2%. Endoleaks occurred in 8.1%, and 7.8% developed aneurysms of the distal aorta or continued FL perfusion with aneurysmal dilation.
Surgery of the descending aorta carries high operative risk. More recently, surgical aortic arch replacement with antegrade stenting of the descending thoracic aorta (‘frozen elephant trunk’) may prove to be a valuable alternative for selected patients.115
12.2 Follow-up after thoracic aortic intervention
For patients undergoing TEVAR or surgical thoracic aortic repair, first follow-up should be performed 1 month after the treatment to exclude the presence of early complications. Surveillance should be repeated after 6 months, 12 months, and then yearly. For patients primarily receiving medical therapy, surveillance should be performed 6 months after initial diagnosis.
12.2.1 Clinical follow-up
Regular clinical follow-up is necessary, more frequently within the first year after diagnosis or intervention and then on a yearly basis. Blood pressure should be monitored closely, as >50% of cases may have resistant hypertension.549 Symptoms of chronic aortic disease are rare and non-specific. New-onset hoarseness or dysphagia may develop with progressive enlargement of the aneurysm. Patients with chronic AD may report symptoms of chronic peripheral malperfusion syndrome (claudication, abdominal pain). Chest or back pain may indicate progression of aortic disease up to (contained) rupture of the aorta.
12.2.2 Imaging after thoracic endovascular aortic repair
For imaging follow-up after TEVAR, CT is the modality of choice. To avoid exposure to radiation, MRI may be more widely used in the future, but is not compatible with stainless steel endografts, due to large artefacts.11 MRI can be safely performed for surveillance of nitinol-based stent-grafts;550 however, it lacks the ability to visualize metallic stent struts and should thus be supplemented by chest X-ray to detect structural disintegration of the metallic stent skeleton. TOE, in combination with chest X-ray, may be used in patients with severe renal dysfunction unable to undergo CT or MRI.
After TEVAR, imaging of the aorta is recommended after 1 month, 6 months, 12 months, and then yearly. If, after TEVAR for TAA, patients show a stable course without evidence of endoleak over 24 months, it may be safe to extend imaging intervals to every 2 years; however, clinical follow-up of the patient́s symptom status and accompanying medical therapy should be maintained at yearly intervals. Patients with TEVAR for AD should receive yearly imaging, since the FL of the abdominal aorta is usually patent and prone to disease progression.
12.2.3 Imaging after thoracic aortic surgery
After aortic surgery, less-strict imaging intervals may be sufficient if a stable course has been documented over the first year. Imaging should focus on surgery-related complications (e.g. suture aneurysm) but should also evaluate disease progression in remote parts of the aorta. After surgery for Type A AD, dissection of the descending and abdominal aorta usually persists and has to be imaged at intervals similar to those described above.
12.3 Follow-up of patients after intervention for abdominal aortic aneurysm
12.3.1 Follow-up after endovascular aortic repair
Computed tomography is the first choice for follow-up imaging after EVAR; however, it is expensive and exposes patients to ionizing radiation and potentially nephrotoxic contrast agent. Duplex ultrasound, with or without contrast agents, is specific for the detection of endoleaks after EVAR.311 A recent meta-analysis showed that the sensitivity and specificity of contrast-enhanced Doppler ultrasonography (DUS) may be superior to Duplex ultrasound alone to detect Type 2 endoleak, which is caused by retrograde flow from side branches and is largely a benign condition that rarely requires secondary intervention.311 Clinically relevant Types 1 and 3 endoleaks, for which re-intervention is required, may be detected with sufficient accuracy with Duplex ultrasound alone and the use of contrast agents has not been shown to be superior in this setting.311
Magnetic resonance imaging has high diagnostic accuracy for detection of endoleaks after EVAR, but is also expensive and cannot visualize the metallic stent struts. It should thus be complemented with plain X-ray for evaluation of the metal stent skeleton. Magnetic resonance imaging is not compatible with stainless steel endografts due to the occurrence of artefacts.
12.3.2 Follow-up after open surgery
All patients should be provided with the best current medical treatment protocol. Post-operative surveillance of open aortic repair may be considered at 5-yearly intervals after open AAA repair to investigate for para-anastomotic aortic aneurysm using colour Doppler ultrasound or CT imaging. Also, patients with AAA appear to have a relatively high risk for incisional hernia. In an observational study using Medicare data, repair of incisional hernia was required in 5.8% of patients within 4 years.
Recommendations for follow-up and management of chronic aortic diseases
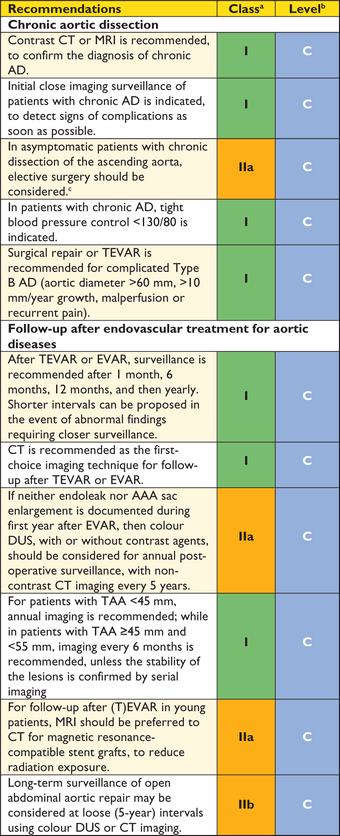 |
 |
aClass of recommendation.
bLevel of evidence.
cPending comorbidities and perioperative risk.
AAA = abdominal aortic aneurysm; AD = aortic dissection; CT = computed tomography; DUS = duplex ultrasonography; EVAR = endovascular aortic repair; MRI = magnetic resonance imaging; TAA = thoracic aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
Recommendations for follow-up and management of chronic aortic diseases
 |
 |
aClass of recommendation.
bLevel of evidence.
cPending comorbidities and perioperative risk.
AAA = abdominal aortic aneurysm; AD = aortic dissection; CT = computed tomography; DUS = duplex ultrasonography; EVAR = endovascular aortic repair; MRI = magnetic resonance imaging; TAA = thoracic aortic aneurysm; TEVAR = thoracic endovascular aortic repair.
13. Gaps in evidence
As illustrated by the large number of ‘level C’ recommendations in this document, the level of evidence for the management of various diseases of the aorta is often weaker than in other cardiovascular conditions. This Task Force emphasizes the need for scientific networking and multicentre trials on several aspects of the management of aortic diseases. The Task Force highlights, briefly, major gaps in evidence that need further research as a priority:
Epidemiological data on the occurrence of AAS are scarce in Europe and globally.
More evidence is needed on the caseload–outcome relationship in the field of aortic diseases.
The implementation and efficacy of aortic centres in Europe should be assessed. The establishment of a European network of aortic centres should be encouraged, along with the establishment of large registries.
Further studies are needed to validate the most accurate, reproducible, and predictive method of measuring the aorta using different imaging modalities.
With the development of 3D imaging and other dynamic imaging methods for the prediction of complications in aneurysmal disease, the superiority of these techniques over 2D size measurement should be assessed.
There is a lack of evidence on the efficacy of medical therapy in chronic aortic diseases (especially chronic AD, TAA, and AAA), particularly regarding antihypertensive drugs and statins.
For TAA, randomized studies are needed on the optimal timing for preventive intervention according to lesion size and other characteristics, as well as individual patient characteristics.
In many cases (e.g. the indication for management of AAA according to its size) the management of women with aortic diseases is based on studies conducted in men. Gender-specific data are essential.
Since the aortic diameter continues to evolve in adulthood, it remains unclear whether the oversizing practice should differ for TEVAR in young patients (e.g. in TAI).
The optimal timing and technique of intervention in chronic AD is still unclear.
14. Appendix
ESC National Cardiac Societies actively involved in the review process of the 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases:
Austria: Austrian Society of Cardiology, Michael Grimm; Azerbaijan: Azerbaijan Society of Cardiology, Oktay Musayev; Belgium: Belgian Society of Cardiology, Agnès Pasquet; Bosnia and Herzegovina: Association of Cardiologists of Bosnia & Herzegovina, Zumreta Kušljugić; Croatia: Croatian Cardiac Society, Maja Cikes; Cyprus: Cyprus Society of Cardiology, Georgios P. Georghiou; Czech Republic: Czech Society of Cardiology, Josef Stasek; Denmark: Danish Society of Cardiology, Henning Molgaard; Estonia: Estonian Society of Cardiology, Sirje Kõvask; Finland: Finnish Cardiac Society, Ville Kytö; France: French Society of Cardiology, Guillaume Jondeau; Georgia: Georgian Society of Cardiology, Zviad Bakhutashvili; Germany: German Cardiac Society, Yskert von Kodolitsch; Greece: Hellenic Cardiological Society, Costas Tsioufis; Hungary: Hungarian Society of Cardiology, András Temesvári; Israel: Israel Heart Society, Ronen Rubinshtein; Italy: Italian Federation of Cardiology, Francesco Antonini-Canterin; Kyrgyzstan: Kyrgyz Society of Cardiology, Olga Lunegova; Latvia: Latvian Society of Cardiology, Peteris Stradins; Lebanon: Lebanese Society of Cardiology, Elie Chammas; Lithuania: Lithuanian Society of Cardiology, Regina Jonkaitiene; Malta: Maltese Cardiac Society, Andrew Cassar; Norway: Norwegian Society of Cardiology, Knut Bjørnstad; Poland: Polish Cardiac Society, Kazimierz Widenka; Portugal: Portuguese Society of Cardiology, Miguel Sousa Uva; Romania: Romanian Society of Cardiology, Daniel Lighezan; Serbia: Cardiology Society of Serbia, Jovan Perunicic; Slovakia: Slovak Society of Cardiology, Juraj Madaric; Spain: Spanish Society of Cardiology, Isidre Vilacosta; Sweden: Swedish Society of Cardiology, Magnus Bäck; Tunisia: Tunisian Society of Cardiology and Cardio-Vascular Surgery, Abdallah Mahdhaoui; Turkey: Turkish Society of Cardiology, Recep Demirbag; Ukraine: Ukrainian Association of Cardiology, Ivan Kravchenko
15. Web addenda
All Web Figures and Web Tables are available in the online addenda at: http://www.escardio.org/guidelines-surveys/esc-guidelines/Pages/aortic-diseases.aspx
References
Author notes
ESC Committee for Practice Guidelines (CPG): Jose Luis Zamorano (Chairperson) (Spain), Stephan Achenbach (Germany), Helmut Baumgartner (Germany), Jeroen J. Bax (Netherlands), Héctor Bueno (Spain), Veronica Dean (France), Christi Deaton (UK), Çetin Erol (Turkey), Robert Fagard (Belgium), Roberto Ferrari (Italy), David Hasdai (Israel), Arno Hoes (The Netherlands), Paulus Kirchhof (Germany/UK), Juhani Knuuti (Finland), Philippe Kolh (Belgium), Patrizio Lancellotti (Belgium), Ales Linhart (Czech Republic), Petros Nihoyannopoulos (UK), Massimo F. Piepoli (Italy), Piotr Ponikowski (Poland), Per Anton Sirnes (Norway), Juan Luis Tamargo (Spain), Michal Tendera (Poland), Adam Torbicki (Poland), William Wijns (Belgium), and Stephan Windecker (Switzerland).
Document reviewers: Petros Nihoyannopoulos (CPG Review Coordinator) (UK), Michal Tendera (CPG Review Coordinator) (Poland), Martin Czerny (Switzerland), John Deanfield (UK), Carlo Di Mario (UK), Mauro Pepi (Italy), Maria Jesus Salvador Taboada (Spain), Marc R. van Sambeek (The Netherlands), Charalambos Vlachopoulos (Greece), and Jose Luis Zamorano (Spain).
The disclosure forms of the authors and reviewers are available on the ESC website www.escardio.org/guidelines
Other ESC entities having participated in the development of this document:
ESC Associations: Acute Cardiovascular Care Association (ACCA), European Association of Cardiovascular Imaging (EACVI), European Association of Percutaneous Cardiovascular Interventions (EAPCI).
ESC Councils: Council for Cardiology Practice (CCP).
ESC Working Groups: Cardiovascular Magnetic Resonance, Cardiovascular Surgery, Grown-up Congenital Heart Disease, Hypertension and the Heart, Nuclear Cardiology and Cardiac Computed Tomography, Peripheral Circulation, Valvular Heart Disease.
The content of these European Society of Cardiology (ESC) Guidelines has been published for personal and educational use only. No commercial use is authorized. No part of the ESC Guidelines may be translated or reproduced in any form without written permission from the ESC. Permission can be obtained upon submission of a written request to Oxford University Press, the publisher of the European Heart Journal and the party authorized to handle such permissions on behalf of the ESC.
Disclaimer: The ESC Guidelines represent the views of the ESC and were produced after careful consideration of the scientific and medical knowledge and the evidence available at the time of their dating.
The ESC is not responsible in the event of any contradiction, discrepancy and/or ambiguity between the ESC Guidelines and any other official recommendations or guidelines issued by the relevant public health authorities, in particular in relation to good use of health care or therapeutic strategies. Health professionals are encouraged to take the ESC Guidelines fully into account when exercising their clinical judgment, as well as in the determination and the implementation of preventive, diagnostic or therapeutic medical strategies; however, the ESC Guidelines do not override, in any way whatsoever, the individual responsibility of health professionals to make appropriate and accurate decisions in consideration of each patient's health condition and in consultation with that patient and, where appropriate and/or necessary, the patient's caregiver. Nor do the ESC Guidelines exempt health professionals from taking full and careful consideration of the relevant official updated recommendations or guidelines issued by the competent public health authorities in order to manage each patient's case in light of the scientifically accepted data pursuant to their respective ethical and professional obligations. It is also the health professional's responsibility to verify the applicable rules and regulations relating to drugs and medical devices at the time of prescription.
National Cardiac Societies document reviewers: listed in the Appendix.



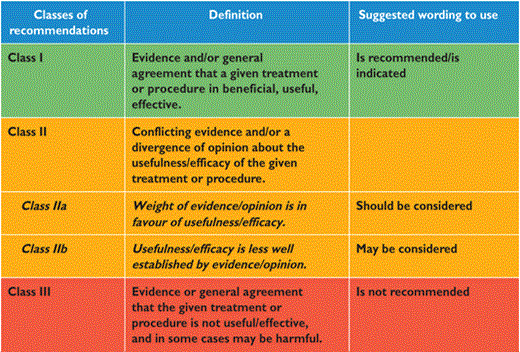
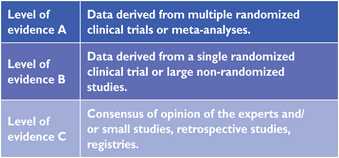
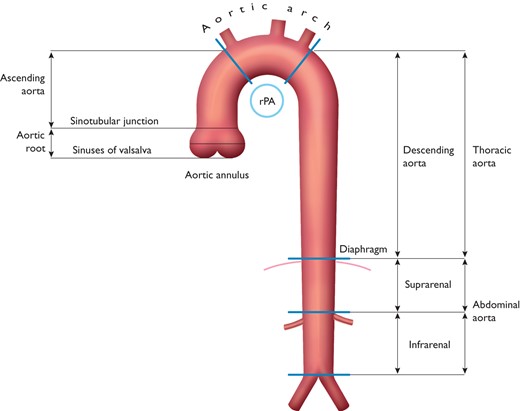
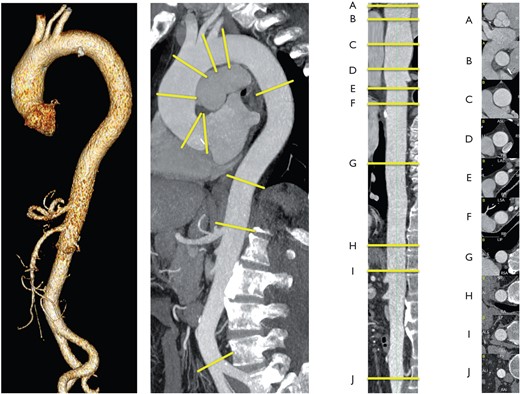
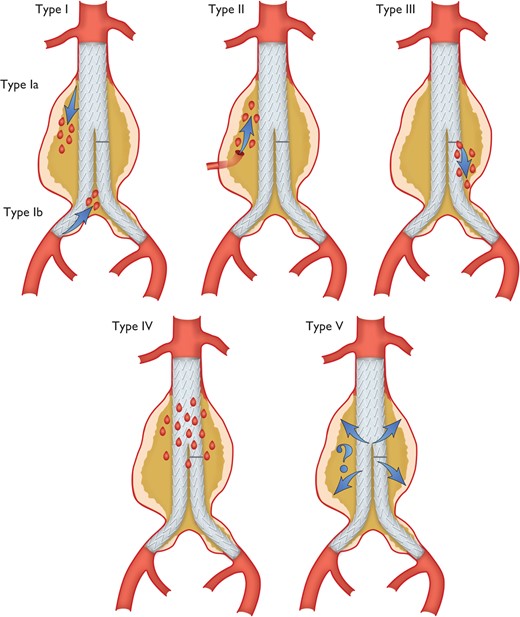
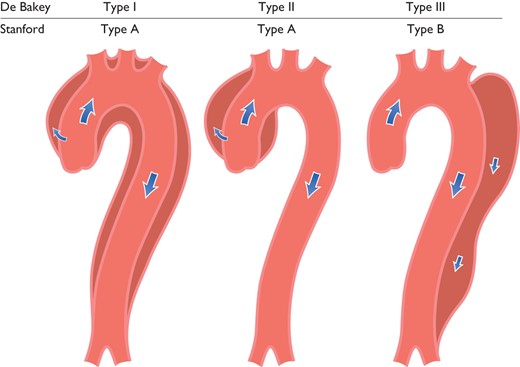
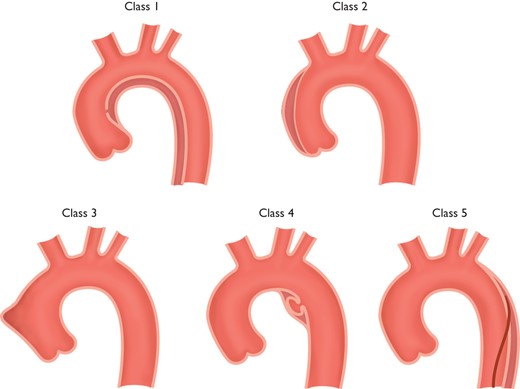
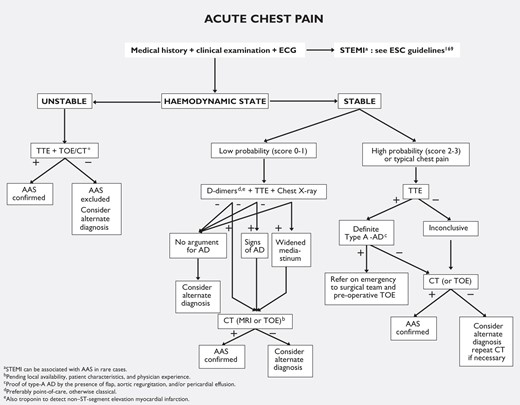
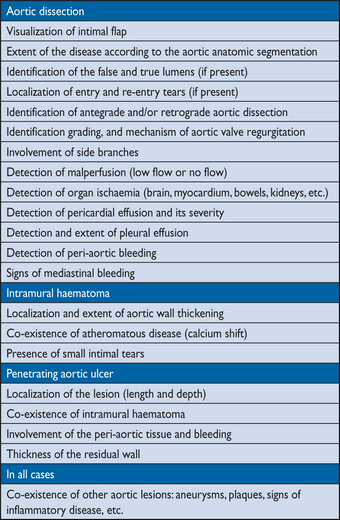
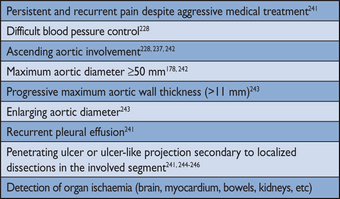

Comments