-
PDF
- Split View
-
Views
-
Cite
Cite
A. L. Herrick, Pathogenesis of Raynaud's phenomenon, Rheumatology, Volume 44, Issue 5, May 2005, Pages 587–596, https://doi.org/10.1093/rheumatology/keh552
Close - Share Icon Share
Abstract
The pathogenesis of Raynaud's phenomenon is not fully understood. However, the last 20 yr have witnessed enormous increases in our understanding of different mechanisms which, singly or in combination, may contribute. A key point is that Raynaud's phenomenon can be either primary (idiopathic) or secondary to a number of underlying conditions, and that the pathogenesis and pathophysiology vary between these conditions. This review concentrates upon those subtypes of Raynaud's phenomenon of most interest to rheumatologists: systemic sclerosis-related Raynaud's phenomenon, primary Raynaud's phenomenon and Raynaud's phenomenon secondary to hand–arm vibration syndrome. In this review, I shall discuss the main mechanisms thought to be important in pathophysiology under the three broad headings of ‘vascular’, ‘neural’ and ‘intravascular’. While these are false distinctions because all interrelate, they facilitate discussion of the key elements: the blood vessel wall (particularly the endothelium), the neural control of vascular tone, and the many circulating factors which can impair blood flow and/or cause endothelial injury. Vascular abnormalities include those of both structure and function. Neural abnormalities include deficiency of the vasodilator calcitonin gene-related peptide (released from sensory afferents), α2-adrenoreceptor activation (possibly with up-regulation of the normally ‘silent’ α2C-adrenoreceptor) and a central nervous system component. Intravascular abnormalities include platelet activation, impaired fibrinolysis, increased viscosity and probably oxidant stress. As our understanding of the pathophysiology of Raynaud's phenomenon increases, so do our possibilities for identifying effective treatments.
Maurice Raynaud described the phenomenon which so famously bears his name in 1862. Summarizing his observations, he stated that ‘local asphyxia of the extremities’ was a result of ‘increased irritability of the central parts of the cord presiding over vascular innervation’ [1]. Approximately 70 yr later, Lewis suggested that the cause of the phenomenon was not central but peripheral, due to ‘spasm of the digital arteries’ and that ‘the abnormal element in the reaction to cold is a direct reaction and due to a peculiar condition of the vessel wall locally: it is not the result of a reflex through the vasomotor nerves’ [2]. Another 70 yr later, the pathophysiology of Raynaud's phenomenon continues to elude us. Despite the evidence suggesting that peripheral mechanisms are primarily responsible in most patients, it seems likely that central mechanisms contribute, especially as ‘Raynaud's’ may affect internal organs. It must be emphasized that Raynaud's is a phenomenon, which can be primary (idiopathic) or secondary to a number of different conditions, including not only rheumatological diseases but also a variety of other causes, including extrinsic vascular obstruction, as in thoracic outlet syndrome, paraproteinaemias and certain drugs/chemicals (e.g. β-blockers, polyvinyl chloride) [3, 4]. The exact pathogenesis and pathophysiology will vary between these conditions.
Raynaud's phenomenon is essentially an exaggerated vasospastic response to cold or to emotion. Classically, the digits turn white (ischaemia), then blue (deoxygenation), then red (reperfusion). In this review, I shall concentrate upon primary Raynaud's phenomenon (PRP) and Raynaud's phenomenon secondary to systemic sclerosis (SSc), with brief mention of hand–arm vibration syndrome (vibration white finger), because these are most relevant to the rheumatologist in terms of understanding pathophysiology. Raynaud's phenomenon secondary to other rheumatological diseases has been less well researched. PRP, although not in itself a serious condition, is of interest to rheumatologists because a key issue is why patients with SSc-spectrum disorders, but not with PRP, go on to develop irreversible digital ischaemia.
It has been suggested that the pathogenesis of Raynaud's phenomenon, especially when secondary to SSc, can be explained on the basis of dysregulated neuroendothelial control mechanisms [5]. The key issue is the imbalance between vasoconstriction and vasodilation (in favour of vasoconstriction). Evidence (which will be reviewed) points to abnormalities in the blood vessel wall (including endothelium, smooth muscle), in the neural control of vascular tone, and in circulating mediators (including those produced as a result of platelet activation and of oxidative stress). For the purposes of this review, these different abnormalities will be broadly considered under the headings of ‘vascular’, ‘neural’ and ‘intravascular’. However, it must be emphasized from the outset that these are false distinctions because all interrelate.
Vascular abnormalities
Structural abnormalities
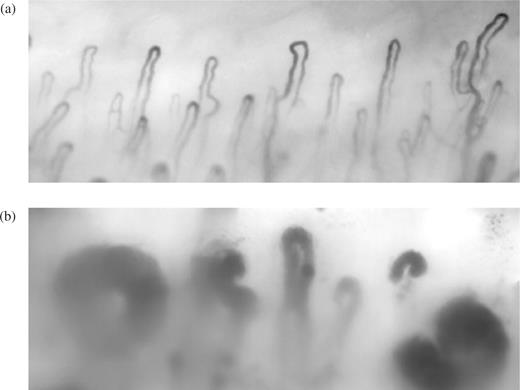
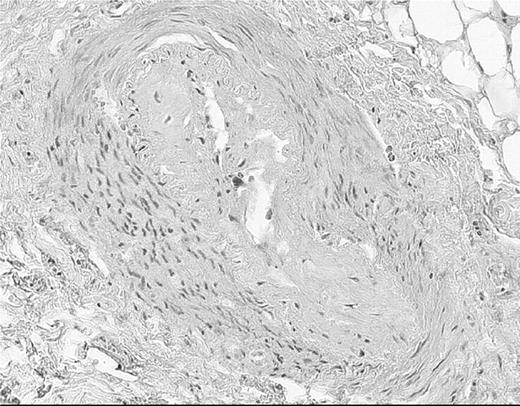
Although subtle microvascular abnormalities may occur in PRP [6], it is generally accepted that in PRP the vascular defect is primarily functional. In contrast, structural vascular abnormalities of both the microvasculature (Fig. 1) and digital artery (Fig. 2) are well recognized in SSc [7–9]. Vascular structure and function in patients with SSc are interdependent. There is no doubt that blood flow in the digits of the patients shown in Figs 1b and 2 is going to be severely compromised, even at ambient temperature, and that any superimposed vasospasm, for example as a result of cold exposure, increases the risk of irreversible tissue damage.
Nailfold capillaries from (a) a healthy control subject and (b) a patient with SSc showing abnormal, widened capillary loops.
A digital artery from a patient with limited cutaneous SSc, showing intimal thickening with breaks in continuity of the internal elastic lamina. The lumen is almost completely occluded. (Figure by courtesy of Professor A. J. Freemont, Manchester, UK.) This figure may be viewed in colour as supplementary data at Rheumatology Online.
The pathogenesis of the structural vascular abnormalities in SSc is not fully understood and is outside the scope of this review; however, it is well discussed elsewhere [9–11] and includes endothelial cell apoptosis, up-regulation of adhesion molecules, and the interplay of a large number of cytokines and growth factors, and pericyte activation [12]. Endothelial abnormalities occur early [13], but it is not understood how these progress to the florid changes demonstrated in Fig. 2 with pronounced intimal thickening, changes so severe as to lead to almost complete vessel occlusion.
Structural vascular abnormalities have also been reported in hand–arm vibration syndrome. Takeuchi et al. described arterial smooth muscle hypertrophy and periarterial fibrosis in a study of digital biopsies [14] and minor structural abnormalities have been reported on nailfold microscopy [15].
Functional abnormalities
This section concentrates upon those functional abnormalities which primarily reflect defects in the endothelium. It is now well recognized that the endothelium is not an inert barrier, but a highly sophisticated layer of cells which reacts to, and produces, a variety of vasoactive substances, including vasoconstrictors and vasodilators. When the endothelium is activated/injured, as in patients with SSc, the balance between vasodilation and vasoconstriction may be disturbed in favour of vasoconstriction (in addition to the endothelium becoming procoagulant and proinflammatory). While it is possible that endothelial activation occurs also in PRP (some investigators have reported raised levels of von Willebrand factor, although others have not [16]), endothelial abnormalities are less likely to be major players in the pathogenesis of PRP.
Impaired vasodilation
This may result from a defect in the endothelium, or reduced concentrations of endogenous vasodilators acting on the endothelium.
Impaired endothelial-dependent vasodilation
In recent years, there has been considerable interest in the hypothesis that in SSc (and possibly also in PRP) endothelial-dependent vasodilation (i.e. vasodilation requiring an intact endothelium) is compromised at an earlier stage of disease than endothelial-independent vasodilation. This area of research has been facilitated by the development of non-invasive techniques for measuring vasodilation in both small and large vessels. Iontophoresis, a method of driving chemicals into the skin using a small electric current, together with measurement of blood flow responses with laser Doppler, assesses the microcirculation. Iontophoresis with acetylcholine chloride is ‘endothelial-dependent’ because it is dependent upon nitric oxide (NO) production within the endothelium. Iontophoresis with sodium nitroprusside is ‘endothelial-independent’ because the nitroprusside acts as an NO donor and the endothelium is effectively bypassed. Ultrasound studies using the technique described by Celermajer et al. [17] assess endothelial-dependent and -independent responses in large vessels (usually the brachial artery).
While studies examining small and large vessel function in SSc have given conflicting results, with some studies showing no impairment of endothelial function even in patients with SSc [18, 19], there is now a considerable weight of evidence from studies using a variety of methods to support a defect in endothelial-dependent vasodilation in SSc [20–26]. Some investigators have reported a defect of endothelial-dependent vasodilation in patients with PRP [27, 28] and Khan et al. reported impairment of both endothelial-dependent and endothelial-independent responses in PRP [29]. Endothelium-dependent and -independent vasodilation have been little studied in hand–arm vibration syndrome.
Reduced production of vasodilators
The endothelium produces a large number of vasodilator substances, including NO and prostacyclin. If the endothelium is damaged, these may be underproduced. It has been suggested that NO deficiency contributes to the pathogenesis of Raynaud's phenomenon, and this has therapeutic relevance because topical application/delivery of NO is known to increase blood flow [30–33]. However, the situation is complex as NO may be overexpressed in SSc [34, 35], and it is possible that NO status in SSc depends on the stage of the disease. A recent study of 20 patients with PRP and 20 with secondary Raynaud's (seven had SSc) reported higher circulating levels of asymmetrical dimethylarginine (an endogenous inhibitor of endothelial NO synthase) and of endothelin-1 in the secondary Raynaud's group [36]. This is an interesting finding which, if confirmed, may further implicate NO in the pathophysiology of Raynaud's phenomenon.
With respect to prostacyclin, a study of rat aortic rings suggested that prostacyclin production is reduced on cold exposure [37]. However, Belch et al. reported that patients with SSc demonstrated increased circulating levels of stable metabolites of prostacyclin, speculating that patients with SSc might be resistant to prostacyclin, although this resistance could be overcome by pharmacological doses [38]. At present, it is not known whether reduced endogenous prostacyclin contributes to the pathogenesis of Raynaud's.
Increased vasoconstriction
The endothelium produces vasoconstrictors as well as vasodilators and the one which has attracted most recent interest in Raynaud's phenomenon is endothelin-1, especially now that it is possible to block its action therapeutically with receptor antagonists. Endothelin-1 is an extremely potent vasoconstrictor with effects on vascular remodelling [39], and also relevant to SSc is that it is profibrotic.
Although a recent study suggested that endothelin-1 levels, at baseline and after a cold challenge, were no different in patients with PRP or SSc and in healthy controls [40], it seems likely that endothelin is implicated in SSc. This is because endothelin-1 is overexpressed in sclerodermatous skin [41] and endothelin-binding density is increased in skin biopsies from patients with SSc [42]. In addition, bosentan (an endothelin-1 receptor antagonist) has been shown to confer benefit in studies of pulmonary arterial hypertension, which included patients with SSc [43, 44] and in a recent preliminary study of SSc-related digital ulceration [45]. There is also some evidence that endothelin-1 may have a role in PRP. Zamora et al. reported that endothelin-1 levels (as well as being elevated at baseline) rose more in patients with PRP than in controls in response to a cold challenge [46], and although Leppert et al. [47] (similarly to Smythe et al. [40]) found that basal endothelin-1 levels were similar in healthy controls and PRP patients, levels rose after whole-body cooling in the PRP but not in the control group. However, Knock et al. did not find any increase in endothelin-binding in biopsies from patients with PRP [42]. Endothelin-1 may also be implicated in hand–arm vibration syndrome [48].
Angiotensin is another vasoactive peptide that has both vasoconstrictor and profibrotic effects. Despite the well-established use of angiotensin-converting enzyme (ACE) inhibitors in scleroderma renal crisis, the exact contribution of the renin–angiotensin system to primary and secondary Raynaud's phenomenon remains unknown. A recent study reported increased circulating levels of angiotensin II in patients with diffuse cutaneous SSc (although not in limited cutaneous SSc) [49]. It is now recognized that ACE inhibitors have effects on endothelial function [50]. The next few years will most likely will see increasing research into the role of the renin–angiotensin system in Raynaud's phenomenon, especially now that angiotensin II receptor antagonists are available and reduced both frequency and severity of attacks in both primary and SSc-related Raynaud's phenomenon in an open-label, randomized study [51].
One of the key issues in Raynaud's phenomenon is why vasoconstriction is cold-induced. New insights are provided by a recent series of studies by Furspan et al. [52], who investigated the role of the protein tyrosine kinase signal transduction pathway in PRP by examining responses in dermal arterioles isolated from forearm skin from patients with PRP and healthy control subjects. Responses to three vasoconstrictors—an α2-adrenergic agonist, serotonin and angiotensin II—were increased in PRP patients but only on cooling, and were reversed by protein tyrosine kinase inhibitors. Tyrosine phosphorylation was increased in PRP patients on cooling to 31°C [52]. The role of this signal transduction pathway in the pathophysiology of Raynaud's phenomenon therefore deserves further study in both primary and secondary Raynaud's.
Conclusions
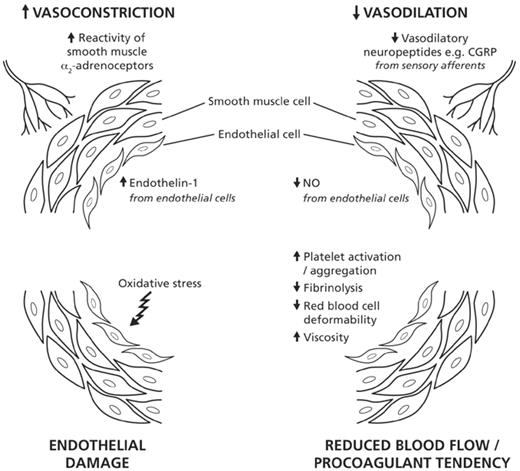
In SSc and hand–arm vibration syndrome, abnormalities in both vascular structure and function interrelate to compromise digital blood flow, whereas in PRP abnormalities are most likely purely functional. In the blood vessel wall, the vasoconstriction versus vasodilator balance is tipped in favour of vasoconstriction by (i) impaired endothelial-dependent vasodilation in SSc, and possibly also in PRP and hand–arm vibration syndrome; and (ii) a mismatch between endothelium-derived vasocontrictors (primarily endothelin-1) and vasodilators (primarily NO and prostacyclin) (Fig. 3). However, there is at present little direct evidence to support this claim other than increased expression of endothelin-1 in SSc and probably also in PRP and hand–arm vibration syndrome.
Further research into signal transduction mechanisms should provide increased insight into the mechanism of cold-induced vasospasm.
Neural abnormalities
Peripheral mechanisms
The sympathetic nervous system is a major player in thermoregulation [53] and it therefore seems likely that the autonomic nervous system contributes to the pathogenesis of both primary and secondary Raynaud's phenomenon via both central and peripheral (local) mechanisms. Until recently, the nervous system in SSc was a neglected area of research. However, autonomic [54, 55] and peripheral [56] neuropathy are being increasingly recognized in SSc [reviewed in 57]. The situation is complex and a variety of neurotransmitters and their receptors are likely to be involved, relating to small sensory nerve fibres as well as to sympathetic vasoconstrictor and vasodilator nerves. Neuropathy is well described in hand–arm vibration syndrome, and paraesthesia is common [14, 58].
Impaired vasodilation
Nerves supplying blood vessels produce a number of vasodilatory substances. Calcitonin gene-related peptide (CGRP), released from sensory afferents, has been most studied: others include substance P, neurokinin A and vasointestinal peptide. Immunohistochemical studies have shown a reduction in the number of CGRP-immunoreactive nerve fibres in biopsies of finger skin from patients with SSc and with PRP (changes in the SSc patients were more marked) [59], and hand–arm vibration syndrome [60]. In the biopsies from patients with hand–arm vibration syndrome (and in a subpopulation of nerves in patients with SSc and PRP [61]) there was also a reduction in the pan-neuronal marker protein gene product 9.5, suggesting that the CGRP depletion might be a consequence of damage to peripheral nerve. Although intravenous CGRP has been reported to improve blood flow in patients with severe Raynaud's phenomenon secondary to connective tissue disease [62], the numbers of patients studied have been small.
Increased vasoconstriction
Several investigators have examined adrenergic function in both primary and secondary Raynaud's phenomenon [63–69]. Our understanding about the different adrenoreceptors has greatly expanded in recent years; there are at least nine subtypes [70]. Vasoconstriction to noradrenaline is mediated via α1 and α2-adrenoreceptors, but the α2-adrenoreceptors are thought to be more important in the regulation of digital vascular tone [65, 66, 71, 72]. Cold augments smooth muscle α2-adrenoreceptor function [73]. There are three subtypes of α2-adrenoreceptors (α2A, α2B and α2C), and it has been recently shown from work in animal models that it is probably the α2C-adrenoreceptor which is the most important in thermoregulation [73]. In a cell model, in response to cooling, the normally ‘silent’ α2C-adrenoreceptors relocate from the Golgi compartments to the cell surface [74]. Therefore, the α2C-adrenoreceptors may be responsible for cold-induced augmentation of α2-adrenoreceptor activity. However, the interrelationships between the different receptor subtypes are highly complex, with the result that it is not yet known whether selective α2C blockade might be an appropriate line of therapy [11].
The balance between α1- and α2-adrenoreceptors differs between proximal and distal arteries: α2-adrenoreceptor responsiveness was increased in digital arteries compared with more proximal vessels [75]. This further supports the hypothesis that increased peripheral expression of α2-adrenoreceptors could contribute to Raynaud's phenomenon. Recent support for the α2-adrenoreceptor playing a key role in the pathogenesis of SSc-related Raynaud's comes from a study of dermal arterioles from uninvolved skin from patients with diffuse SSc; arteriolar smooth muscle demonstrated increased α2-adrenoreceptor reactivity compared with controls, and this increased reactivity was endothelium-independent [69]. α2-Adrenoreceptors may also be implicated in hand–arm vibration syndrome [63].
Central mechanisms
Many patients with Raynaud's report stress-induced vasospasm, and it therefore seems intuitive that even if neural abnormalities are primarily local to the digits, there must also be a central nervous component. However, this is a difficult area of research, and there is little direct evidence in support of central mechanisms. Edwards et al. showed that patients with PRP do not habituate in the same way as healthy controls to the components of the ‘alerting response’ evoked by acute emotional stress (a sound stimulus) [76, 77]. This alerting response includes vasodilation in forearm muscle and vasoconstriction in the cutaneous circulation of the digits. Digital cutaneous vasoconstriction in response to a cool stimulus was prolonged in the PRP patients and was associated with a rise in endothelin-1 levels in venous blood taken from the dorsum of the cooled hand [77]. The authors suggested that while central nervous mechanisms prolonged vasoconstriction, there was a superimposed effect of locally released endothelin-1.
Conclusions
Release of neurotransmitters from both autonomic and sensory afferent nerves contributes to digital vascular tone. In patients with primary and secondary Raynaud's phenomenon, key factors in neural control mechanisms that are likely to be tipping the vasoconstriction vs vasodilator balance in favour of vasoconstriction are: (i) impaired vasodilation, including that due to CGRP deficiency; (ii) increased vasoconstriction mediated by activation of α2-adrenoreceptors (Fig. 3). This activation is augmented by cold exposure. It may be that in patients with Raynaud's phenomenon there is a lower (i.e. higher temperature) threshold for α2C-adrenoreceptors to become activated than in healthy controls.
Central mechanisms may contribute to vasospasm but in most situations peripheral neural mechanisms are the more important.
Intravascular abnormalities
Many circulating factors have been implicated in the pathogenesis of Raynaud's phenomenon, especially in patients with SSc. These include platelet activation, impaired fibrinolysis, white blood cell activation, reduced red blood cell deformability and oxidative stress, all of which have been described in subsets of patients with Raynaud's. Often it is not clear whether the abnormality is a primary or a secondary event. However, there is no doubt that in some patients circulating factors are all important, evidenced by cryoglobulinaemia being a ‘cause’ of Raynaud's phenomenon.
Below are discussed some of the intravascular factors implicated in the pathophysiology of Raynaud's. Other possible contributors include shear stress (which could equally well be considered under the ‘vascular’ heading) [78] and antiendothelial cell antibodies (via endothelial cell activation) [79]. However, these will not be described further—at present their roles in the pathophysiology of Raynaud's phenomenon are unclear.
Platelet activation
Platelet activation is well recognized in SSc [80–84] and PRP [16, 81, 83, 84], and vibration may cause platelet activation in both healthy controls and patients with hand–arm vibration syndrome [85]. Platelet activation can be demonstrated by increased circulating levels of thromboxane and of β-thromboglobulin, released from platelet α-granules. Synthesis of thromboxane, a potent vasoconstrictor as well as a platelet aggregator, is increased in patients with SSc, especially on cooling [86], and so may contribute to vasospasm in SSc, and expression of the gene encoding thromboxane synthase was recently found to be increased in leucocytes from patients with SSc [87]. Serotonin, another vasoconstrictor produced from platelets, has also been implicated in the pathophysiology of Raynaud's [88].
Fibrinolysis
In patients with PRP, fibrinolysis is probably normal [16, 89]. However, defective fibrinolysis has been reported in SSc [16, 84, 90]. For example, elevated circulating levels of tissue plasminogen activator antigen, which is a product of endothelial cells and which might therefore be released in states of endothelial activation, have been reported in SSc [16, 91], and other investigators have found raised levels of tissue plasminogen activator inhibitor [90]. The situation is complex and results are conflicting, but the conclusion is that at least a proportion of patients with SSc have impaired fibrinolysis, which will predispose towards fibrin deposition and vascular obstruction. Ames et al. reported abnormalities of coagulation as well as of fibrinolysis in patients with SSc [90].
White blood cell activation
White blood cell activation has been reported in patients with SSc, PRP and hand–arm vibration syndrome [92, 93] and may contribute to oxidative stress, described below.
Reduced red blood cell deformability
This has been reported in SSc, but not in PRP [38, 94] and may reflect damage to the erythrocyte membrane by free radicals [95]. Those familiar with the very low capillary flow rates in many patients with SSc on nailfold video microscopy will be able to envisage easily how any reduction in red blood cell deformability might further compromise the microvasculature.
Increased viscosity
Increased blood viscosity has long been recognized in patients with Raynaud's phenomenon. Goyle et al. found that viscosity was increased in patients with PRP at low temperatures [96]. Picart et al. reported that increased blood viscosity in patients with SSc was associated with low flow rates, but that viscosity did not differ between patients with PRP and healthy controls [97]. Increased viscosity has also been implicated in hand–arm vibration syndrome [98, 99].
Oxidative stress
Episodes of Raynaud's phenomenon, if severe, might be expected to cause repeated episodes of ischaemic reperfusion injury, and oxidative stress (mediated by free radicals) has been implicated in the pathogenesis of Raynaud's phenomenon and SSc [100, 101]. Free radicals may be produced by a variety of mechanisms, including the hypoxanthine–xanthine oxidase system and activation of polymorphonuclear leucocytes, as already mentioned above. Oxidative stress has been less studied in PRP than in SSc, but a cross-sectional study including patients with SSc and PRP showed some elevation of free radical markers and reduced levels of ascorbic acid, an antioxidant, in both groups, suggesting that oxidative stress may also occur in PRP [102].
In SSc, it seems likely that oxidative stress contributes to endothelial injury through peroxidation of cell membrane lipids. Further support for a role for oxidative damage in the vascular pathology of SSc comes from the finding of Bruckdorfer et al. that low-density lipoproteins (LDL) in patients with SSc demonstrate increased susceptibility to oxidation compared with LDL from healthy controls or from patients with PRP [103]. Also, increased concentrations of antibodies to oxidized LDL have been reported in SSc [104, 105]. Therefore, a vicious cycle of vasospasm (perhaps against the background of a structurally abnormal vessel), free-radical generation, endothelial injury and more vasospasm can be envisaged.
Conclusions
A wide variety of intravascular or circulating factors has been implicated in the pathophysiology of Raynaud's phenomenon, especially when this is associated with SSc or hand–arm vibration syndrome, in which platelet activation, defective fibrinolysis, reduced red blood cell deformability and increased blood viscosity have all been reported. White blood cell activation and oxidant stress have been reported in PRP as well as in SSc and hand–arm vibration syndrome.
We do not understand how these different factors might interrelate with each other or with the vascular and neural abnormalities already described. It is likely that intravascular factors compromise basal blood flow, especially in the microcirculation, and this is then further compromised by digital vasospasm.
Other factors
Smoking
In patients with SSc, there is an association, between cigarette smoking and the severity of digital ischaemia [106], and although the mechanisms behind this association are not clear, they are likely to include endothelial damage caused by smoking, increased viscosity, impaired fibrinolysis and the high free radical content of cigarette smoke. Smoking may influence hand–arm vibration syndrome: in a study of 601 subjects, smoking was associated with lower finger systolic pressures [107]. In contrast, a large epidemiological study suggested that there was no association between Raynaud's phenomenon and cigarette consumption [108]. It may be that smoking is associated with the severity of Raynaud's (especially in those with underlying vascular disease) rather than susceptibility.
Hormonal factors
Raynaud's phenomenon is much more common in women than in men and it is likely that hormonal factors are important, especially as variations in blood flow with the menstrual cycle have been described: during the immediate preovulatory period, healthy controls demonstrated digital vascular reactivity similar to that of patients with Raynaud's phenomenon [109]. At present we do not fully understand the hormonal contribution to vascular tone.
Genetic factors
These have been implicated in both PRP [110, 111] and SSc [112, 113]. The recent developments in the genetics of pulmonary artery hypertension [114, 115] are of interest. Patients with SSc-related pulmonary arterial hypertension often have severe Raynaud's and therefore understanding the molecular mechanisms whereby mutations lead to vascular pathology may provide insights into Raynaud's phenomenon as well as into pulmonary hypertension.
Implications for treatment
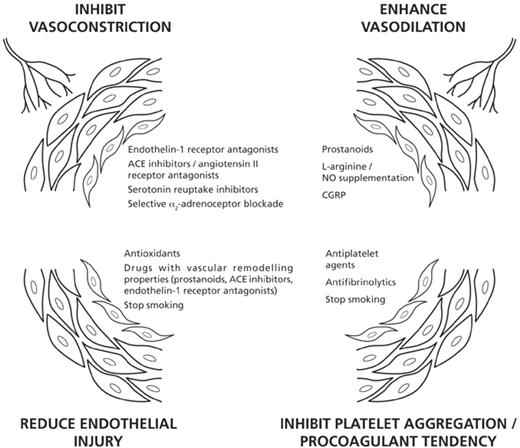
While this review is concerned with pathophysiology rather than treatment, included is a brief consideration of how greater insights into the pathogenesis of Raynaud's phenomenon are directing new avenues of treatment (Fig. 4).
Present and future approaches to the treatment of Raynaud's phenomenon.
Inhibiting vasoconstriction
As already mentioned above, the endothelin-1 receptor antagonist bosentan has recently been shown to confer benefit in patients with SSc-related digital ulceration [45] and further clinical trials are under way. ACE inhibitors have so far been studied little in Raynaud's phenomenon, but there is currently increased interest in ACE inhibitors [116], especially now that their effects on endothelial function [50] and vascular remodelling are recognized [117], and also in angiotensin II receptor antagonists [51]. The action of serotonin can be blocked by serotonin reuptake inhibitors and results from an open study suggest that fluoxetine, a selective serotonin reuptake inhibitor, may be beneficial in both primary and secondary Raynaud's [118]. It is possible that in the future selective blockade of α2-adrenoreceptors could be a potentially exciting new avenue of therapy [73].
Increasing vasodilation
Intravenous prostanoid therapy is well established in severe digital ischaemia [119, 120] and oral prostanoids require further evaluation, especially as prostanoids are now thought also to have vascular remodelling effects [121]. Supplementation of the l-arginine/NO pathway is being explored in several ways, including with transdermal NO [31–33], oral administration of l-arginine, and via phosphodiesterase inhibition (which enhances the effect of NO by inhibiting the degradation of cyclic guanosine monophosphate).
Antithrombotics
This is a neglected area of therapeutics in patients with Raynaud's and more research is required, especially into antiplatelet agents in patients with SSc. Prostanoids, already mentioned, have antiplatelet effects.
Endothelium protection
Several of the above strategies should protect the endothelium. In addition, we need to continue to research antioxidant therapies [122, 123]. Smoking cessation should be actively promoted. Therefore the next 10–15 yr should witness increased understanding of how best to treat Raynaud's phenomenon, possibly using different classes of drug in combination.
Conclusions
Studying Raynaud's phenomenon presents major challenges for several reasons. First, it must be recognized that Raynaud's is a symptom complex of a variety of different conditions which themselves have different pathogeneses. Secondly, vascular physiology is itself tremendously complex, and there is considerable intra- as well as interindividual variation in the control of vascular tone even in healthy subjects.
Many different mechanisms have now been implicated in the pathophysiology of Raynaud's phenomenon. Many of these support the ‘local fault’ hypothesis of Lewis, but others, including intravascular factors and a central nervous component of vasoconstriction, are more in favour of ‘central’ mechanisms, depending upon how these are defined. Contributors to Raynaud's phenomenon include increased vasospasm and reduced vasodilation, structural abnormality of large and small vessels, and coagulopathy. In turn, the major players influencing these mechanisms include endothelin-1, NO, CGRP (and the balance between these), the α2-adrenoreceptor, free radicals (causing oxidant stress) and platelet activation/aggregation. Progress in unravelling the pathophysiology of Raynaud's phenomenon is driving new approaches to treatment.
The author undertakes consultancy work for Actelion.
References
Raynaud M.
Lewis T. Experiments relating to the peripheral mechanism involved in spasmodic arrest of the circulation in the fingers, a variety of Raynaud's disease.
Al-Allaf A-W, Belch JJF. Raynaud's phenomenon. In: Hochberg MC, Silman AJ, Smolen JS et al., eds.
Kaheleh B, Matucci-Cerinic M. Raynaud's phenomenon and scleroderma. Dysregulated neuroendothelial control of vascular tone.
Bukhari M, Herrick AL, Moore T, Manning J, Jayson MIV. Increased nailfold capillary dimensions in primary Raynaud's phenomenon and systemic sclerosis.
Campbell PM, LeRoy EC. Pathogenesis of systemic sclerosis: a vascular hypothesis.
Rodnan GP, Myerowitz RL, Justh GO. Morphological changes in the digital arteries of patients with progressive systemic sclerosis (scleroderma) and Raynaud's phenomenon.
Kahaleh MB, LeRoy EC. Autoimmunity and vascular involvement in systemic sclerosis (SSc).
Flavahan NA, Flavahan S, Mitra S, Chotani MA. The vasculopathy of Raynaud's phenomenon and scleroderma.
Rajkumar VS, Sundberg C, Abraham DJ, Rubin K, Black CM. Activation of microvascular pericytes in autoimmune Raynaud's phenomenon and systemic sclerosis.
Prescott RJ, Freemont AJ, Jones CJP, Hoyland J, Fielding P. Sequential dermal microvascular and perivascular changes in the development of scleroderma.
Takeuchi T, Futatsuka M, Imanishi H, Yamada S. Pathological changes observed in the finger biopsy of patients with vibration-induced white finger.
Littleford RC, Khan F, Hindley MO, Ho M, Belch JJF. Microvascular abnormalities in patients with vibration white finger.
Herrick AL, Illingworth K, Blann A, Hay CRM, Hollis S, Jayson MIV. Von Willebrand factor, thrombomodulin, thromboxane, β-thromboglobulin and markers of fibrinolysis in primary Raynaud's phenomenon and systemic sclerosis.
Celermajer DS, Sorenson KE, Gooch VM et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis.
Anderson ME, Hollis S, Moore T, Jayson MIV, Herrick AL. Non-invasive assessment of vascular reactivity in forearm skin of patients with primary Raynaud's phenomenon and systemic sclerosis.
Anderson ME, Campbell F, Hollis S, Moore T, Jayson MIV, Herrick AL. Non-invasive assessment of digital vascular reactivity in patients with primary Raynaud's phenomenon and systemic sclerosis.
Matucci-Cerinic M, Pietrini U, Marabini S. Local venomotor response to intravenous infusion of substance P and glyceryl trinitrate in systemic sclerosis.
Lekakis J, Mavrikakis M, Papamicheal C et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud's phenomenon.
Freedman RF, Girgis R, Mayes MD. Endothelial and adrenergic dysfunction in Raynaud's phenomenon and scleroderma.
Khan F, Belch JJF. Skin blood flow in patients with systemic sclerosis and Raynaud's phenomenon: effects of oral l-arginine supplementation.
Anderson ME, Moore TL, Hollis S, Clark S, Jayson MI, Herrick AL. Endothelial-dependent vasodilation is impaired in patients with systemic sclerosis, as assessed by low dose iontophoresis.
Anderson ME, Moore TL, Lunt M, Herrick AL. Digital iontophoresis of vasoactive substances as measured by laser Doppler imaging – a non-invasive technique by which to measure microvascular dysfunction in Raynaud's phenomenon.
Bedarida GV, Kim D, Blaschke TF, Hoffman BB. Venodilation in Raynaud's disease.
Smith PJW, Ferro CJ, McQueen DS, Webb DJ. Impaired cholinergic dilator response of resistance arteries isolated from patients with Raynaud's disease.
Khan F, Litchfield SJ, McLaren M, Veale DJ, Littleford RC, Belch JJF. Oral l-arginine supplementation and cutaneous vascular responses in patients with primary Raynaud's phenomenon.
Teh LS, Manning J, Moore T, Tully MP, O'Reilly D, Jayson MIV. Sustained-release transdermal glyceryl trinitrate patches as a treatment for primary and secondary Raynaud's phenomenon.
Khan F, Greig IR, Newton DJ, Butler AR, Belch JJF. Skin blood flow after transdermal S-nitrosothio-acetylglucose.
Tucker AT, Pearson RM, Cooke ED, Benjamin N. Effect of nitric-oxide-generating system on microcirculatory blood flow in skin in patients with severe Raynaud's syndrome: a randomised trial.
Anderson ME, Moore TL, Hollis S, Jayson MIV, King TA, Herrick AL. Digital vascular response to topical glyceryl trinitrate, as measured by laser Doppler imaging, in primary Raynaud's phenomenon and systemic sclerosis.
Yamamoto T, Katayama I, Nichioka K. Nitric oxide production and inducible nitric oxide synthase expression in systemic sclerosis.
Cotton SA, Herrick AL, Jayson MIV, Freemont AJ. Endothelial expression of nitric oxide synthases and nitrotyrosine in systemic sclerosis skin.
Rajagopalan S, Pfenninger D, Kehrer C et al. Increased asymmetric dimethylarginine and endothelin 1 levels in secondary Raynaud's phenomenon. Implications for vascular dysfunction and progression of disease.
Jeremy JY, Mikhailidis DP, Hutton RA, Dandona P. The effect of cooling on in vitro vascular prostacyclin and platelet thromboxane A2 synthesis: relevance to cold-induced pathology.
Belch JJF, McLaren M, Anderson J et al. Increased prostacyclin metabolites and decreased red cell deformability in patients with systemic sclerosis and Raynaud's syndrome.
Kirchengast M, Munter K. Endothelin-1 and endothelin receptor antagonists in cardiovascular remodeling.
Smythe AE, Bell AL, Bruce IN, McGrann S, Allen JA. Digital vascular responses and serum endothelin-1 concentrations in primary and secondary Raynaud's phenomenon.
Vancheeswaran R, Azam A, Black C, Dashwood MR. Localization of endothelin-1 and its binding sites in scleroderma skin.
Knock GA, Terenghi G, Bunker CB, Bull HA, Dowd PM, Polak JM. Characterization of endothelin-binding sites in human skin and their regulation in primary Raynaud's phenomenon and systemic sclerosis.
Channick RN, Simonneau G, Sitbon O et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study.
Rubin LJ, Badesch DB, Barst RJ et al. Bosentan therapy for pulmonary arterial hypertension.
Korn JH, Mayes M, Matucci-Cerinic M et al. Digital ulcers in systemic sclerosis – prevention by treatment with bosentan, and oral endothelin receptor antagonist.
Zamora MR, O'Brien RF, Rutherford RB, Weil JV. Serum endothelin-1 concentrations and cold provocation in primary Raynaud's phenomenon.
Leppert J, Ringqvist A, Karlberb BE, Ringquist I. Whole-body cooling increases plasma endothelin-1 levels in women with primary Raynaud's phenomenon.
Palmer KT, Mason H. Serum endothelin concentrations in workers exposed to vibration.
Kawaguchi Y, Takagi K, Hara M et al. Angiotensin II in the lesional skin of systemic sclerosis patients contributes to tissue fibrosis via angiotensin II type 1 receptors.
Mancini GB, Henry GC, Macaya C et al. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (Trial on Reversing ENdothelial Dysfunction) Study.
Dziadzio M, Denton CP, Smith R et al. Losartan therapy for Raynaud's phenomenon and scleroderma: clinical and biochemical findings in a fifteen-week, randomized, parallel-group, controlled trial.
Furspan PB, Chatter S, Freedman RR. Increased tyrosine phosphorylation mediates the cooling-induced contraction and increased vascular reactivity of Raynaud's disease.
Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why.
Klimiuk PS, Taylor L, Baker RD, Jayson MIV. Autonomic neuropathy in systemic sclerosis.
Dessein PH, Joffe BJ, Metz RM, Millar DL, Lawson M, Stanwix AE. Autonomic dysfunction in systemic sclerosis: sympathetic overactivity and instability.
Schady W, Sheard A, Hassell A, Holt L, Jayson MIV, Klimiuk P. Peripheral nerve dysfunction in scleroderma.
Herrick AL.
Noel B. Pathophysiology and classification of the vibration white finger.
Bunker CB, Terenghi G, Springall DR, Polak JM, Dowd PM. Deficiency of calcitonin gene-related peptide in Raynaud's phenomenon.
Goldsmith PC, Molina FA, Bunker CB et al. Cutaneous nerve fibre depletion in vibration white finger.
Terengi G, Bunker CB, Liu YF et al. Image analysis quantification of peptide-immunoreactive nerves in the skin of patients with Raynaud's phenomenon and systemic sclerosis.
Bunker CB, Reavley C, O'Shaughnessy DJ, Dowd PM. Calcitonin gene-related peptide in treatment of severe peripheral vascular insufficiency in Raynaud's phenomenon.
Ekenvall L, Lindblad LE. Is vibration white finger a primary sympathetic nerve injury?
Coffman JD, Cohen RA. α2-Adrenergic and 5-HT2 receptor hypersensitivity in Raynaud's phenomenon.
Freedman RR, Moten M, Migaly P, Mayes M. Cold-induced potentiation of α2-adrenergic vasoconstriction in primary Raynaud's disease.
Freedman RR, Baer RP, Mayes MD. Blockade of vasospastic attacks by alpha2-adrenergic but not alpha1-adrenergic antagonists in idiopathic Raynaud's disease.
Cooke JP, Creager SJ, Scales KM et al. Role of digital artery adrenoceptors in Raynaud's disease.
Freedman RF, Girgis R, Mayes MD. Endothelial and adrenergic dysfunction in Raynaud's phenomenon and scleroderma.
Flavahan NA, Flavahan S, Liu Q et al. Increased α2-adrenergic constriction of isolated arterioles in diffuse scleroderma.
Coffman JD, Cohen RA. Role of alpha-adrenoceptor subtypes mediating sympathetic vasoconstriction in human digits.
Ekenvall L, Lindblad LE, Norbeck O, Etzell B-M. α-Adrenoceptors and cold-induced vasoconstriction in human finger skin.
Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent α2-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries.
Jeyaraj SC, Chotani MA, Mitra S, Gregg HE, Flavahan NA, Morrison KJ. Cooling evokes redistribution of α2C-adrenoceptors from Golgi to plasma membrane in transfected human embryonic kidney 293 cells.
Flavahan NA, Cooke JP, Shepherd JT, Vanhoutte PM. Human postjunctional alpha-1 and alpha-2 adrenoceptors: differential distribution in arteries of the limbs.
Edwards CM, Marshall JM, Pugh M. Lack of habituation of the pattern of cardiovascular response evoked by sound in subjects with primary Raynaud's disease.
Edwards CM, Marshall JM, Pugh M. Cardiovascular responses evoked by mild cool stimuli in primary Raynaud's disease: the role of endothelin.
Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress-mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses?
Carvalho D, Savage CO, Black CM, Pearson JD. IgG antiendothelial cell antibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokines.
Kahaleh MB, Osborn I, LeRoy EC. Elevated levels of circulating platelet aggregates and beta-thromboglobulin in scleroderma.
Kallenberg CGM, Vellenga E, Wouda AA, The TH. Platelet activation, fibrinolytic activity and circulating immune complexes in Raynaud's phenomenon.
Seibold JR, Harris JN. Plasma β-thromboglobulin in the differential diagnosis of Raynaud's phenomenon.
Lau CS, McLaren M, Saniabadi A, Belch JJF. Increased whole blood platelet aggregation in patients with Raynaud's phenomenon with or without systemic sclerosis.
Silveri F, De Angelis R, Poggi A et al. Relative roles of endothelial cell damage and platelet activation in primary Raynaud's phenomenon (RP) and RP secondary to systemic sclerosis.
Kent PJ, Williams GA, Kester RC. Platelet activation during hand vibration.
Reilly IA, Roy L, Fitzgerald GA. Biosynthesis of thromboxane in patients with systemic sclerosis and Raynaud's phenomenon.
Young V, Ho M, Vosper H, Belch JJF, Palmer CNA. Elevated expression of the genes encoding TNF-α and thromboxane synthase in leucocytes from patients with systemic sclerosis.
Klimiuk PS, Grennan A, Weinkove C, Jayson MIV. Platelet serotonin in systemic sclerosis.
Lau CS, McLaren M, Mackay I, Belch JJF. Baseline plasma fibrinolysis and its correlation with clinical manifestations in patients with Raynaud's phenomenon.
Ames PR, Lupoli S, Alves J et al. The coagulation/fibrinolytic balance in systemic sclerosis: evidence for a haematological stress syndrome.
Marasini B, Cugno M, Bassani C, Stanzani M, Bottasso B, Agostoni A. Tissue-type plasminogen activator and von Willebrand factor plasma levels as markers of endothelial involvement in patients with Raynaud's phenomenon.
Lau CS, O'Dowd A, Belch JJF. White blood cell activation in Raynaud's phenomenon of systemic sclerosis and vibration white finger.
Lau CS, Bridges AB, Muir A, Scott N, Bancroft A, Belch JJF. Further evidence of increased polymorphonuclear cell activity in patients with Raynaud's phenomenon.
Rustin MHA, Kovacs IB, Sowemimo-Coker SO, Maddison PJ, Kirby JDT. Differences in red cell behaviour between patients with Raynaud's phenomenon and systemic sclerosis and patients with Raynaud's disease.
Solans R, Motta C, Sola R et al. Abnormalities of erythrocyte membrane fluidity, lipid composition, and lipid peroxidation in systemic sclerosis.
Picart C, Carpentier PH, Brasseur S, Galliard H, Piau JM. Systemic sclerosis: blood rheometry and laser Doppler imaging of digital cutaneous microcirculation during local cold exposure.
Okada A, Inaba R, Furono T, Nohara S, Ariizumi M. Usefulness of blood parameters, especially viscosity, for the diagnosis and elucidation of pathogenic mechanisms of the hand–arm vibration syndrome.
Greenstein D, Kester RC. The hemorheologic effects of hand-transmitted vibration.
Murrell DF. A radical proposal for the pathogenesis of scleroderma.
Herrick AL, Matucci Cerinic M. The emerging problem of oxidative stress and the role of antioxidants in systemic sclerosis.
Herrick AL, Rieley F, Schofield D, Hollis S, Braganza JM, Jayson MIV. Micronutrient antioxidant status in patients with primary Raynaud's phenomenon and systemic sclerosis.
Bruckdorfer KR, Hillary JB, Bunce T, Vancheeswaran R, Black CM. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis.
Simonini G, Matucci Cerinic M, Generini S et al. Oxidative stress in systemic sclerosis.
Herrick AL, Illingworth KJ, Hollis S, Gomez-Zumaquero JM, Tinahones FJ. Antibodies against oxidized low-density lipoproteins in systemic sclerosis.
Harrison BJ, Silman AJ, Hider SL, Herrick AL. Cigarette smoking: a significant risk factor for digital vascular diseases in patients with systemic sclerosis.
Cherniack M, Clive J, Seidner A. Vibration exposure, smoking, and vascular dysfunction.
Palesch YY, Valter I, Carpentier PH, Maricq HR. Association between cigarette and alcohol consumption and Raynaud's phenomenon.
Lafferty K, de Trafford JC, Potter C, Roberts VC, Cotton LT. Reflex vascular responses in the finger to contralateral thermal stimuli during the normal menstrual cycle: a hormonal basis to Raynaud's phenomenon?
Freedman RR, Mayes MD. Familial aggregation of primary Raynaud's disease.
Susol E, MacGregor AJ, Barrett JH et al. A two-stage, genome-wide screen for susceptibility loci in primary Raynaud's phenomenon.
Arnett FC, Cho M, Chatterjee S, Aguilar MB, Reveille JD, Mayes MD. Familial occurrence and relative risks for systemic sclerosis (scleroderma) in three United States cohorts.
Zhou X, Tan FK, Wang N et al. Genome-wide association study for regions of systemic sclerosis susceptibility in a Choctaw Indian population with high disease prevalence.
Newman JH, Wheeler L, Lane KB et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred.
Trembath RC, Thompson JR, Machado RD et al. Clinical and molecular genetics of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia.
Maddison P. Prevention of vascular damage in scleroderma with angiotensin-converting enzyme (ACE) inhibition.
Weber KT, Brilla CG, Janicki JS. Cardioreparation with lisinopril in the management of hypertension and heart failure.
Coleiro B, Marshall SE, Denton CP et al. Treatment of Raynaud's phenomenon with the selective serotonin reuptake inhibitor fluoxetine.
Wigley FM, Wise RA, Seibold JR et al. Intravenous iloprost infusion in patients with Raynaud phenomenon secondary to systemic sclerosis. A multicenter, placebo-controlled, double-blind study.
Pope J, Fenlon D, Thompson A et al.
Denton CP, Bunce TD, Dorado MB et al. Probucol improves symptoms and reduces lipoprotein oxidation susceptibility in patients with Raynaud's phenomenon.




Comments