-
PDF
- Split View
-
Views
-
Cite
Cite
Hadas Stiebel-Kalish, Eyal Robenshtok, Murat Hasanreisoglu, David Ezrachi, Ilan Shimon, Leonard Leibovici, Treatment Modalities for Graves’ Ophthalmopathy: Systematic Review and Metaanalysis, The Journal of Clinical Endocrinology & Metabolism, Volume 94, Issue 8, 1 August 2009, Pages 2708–2716, https://doi.org/10.1210/jc.2009-0376
Close - Share Icon Share
Background: Graves’ ophthalmopathy (GO) is a common cause of morbidity in patients with Graves’ disease. Optimal treatment of GO remains unclear, and an evidence-based approach may improve patients’ outcome.
Methods: A systematic review and metaanalysis of randomized, controlled trials comparing treatment modalities for GO vs. placebo, no intervention, or other treatments. Primary outcome was the clinical activity score (CAS).
Results: Thirty-three trials evaluating 1367 patients fulfilled inclusion criteria. In patients with moderate to severe GO, iv pulse corticosteroids were significantly better than oral corticosteroids in reducing CAS [standardized mean difference −0.64, 95% confidence interval (CI) −1.11 to −0.17, χ2 7.91, I2 62%, random effect], with lower rate of adverse events. Somatostatin analogs showed a minor but statistically significant advantage over placebo (mean difference −0.63, 95% CI −0.98 to −0.28). There was no advantage of orbital radiotherapy over sham radiation in CAS, but radiotherapy was superior for response rates of diplopia (odds ratio 4.88, 95% CI 1.93–12.34, two trials). Treatment with combination of orbital radiotherapy and corticosteroids was significantly better than with either treatment alone (standardized mean difference −1.05, 95% CI −1.62 to −0.48).
Conclusions: Current evidence demonstrates the efficacy of iv corticosteroids in decreasing CAS in patients with moderate to severe GO. Intravenous pulse corticosteroids therapy has a small but statistically significant advantage oral therapy and causes significantly fewer adverse events. Somatostatin analogs have marginal clinical efficacy. The efficacy of orbital radiotherapy as single therapy remains unclear, whereas the combination of radiotherapy with corticosteroids has better efficacy than either radiotherapy or oral corticosteroids alone.
Graves’ ophthalmopathy (GO), or more accurately thyroid-associated ophthalmopathy, is a common cause of morbidity and discomfort in patients with Graves’ disease. Approximately 20–25% of patients with Graves’ hyperthyroidism have clinically apparent GO at the time of diagnosis (1). Many more patients have evidence of ophthalmopathy on ultrasonography, computed tomography, or magnetic resonance imaging of the orbits (2, 3).
GO may result in eyelid retraction, proptosis, chemosis, periorbital edema, and altered ocular motility. Severe GO leads to exposure keratopathy, diplopia, and compressive optic neuropathy, which might cause visual loss. Gerding et al. (4) evaluated quality of life in patients with GO and demonstrated low scores in the categories of physical functioning, social functioning, mental health, health perceptions, and bodily pain in this group.
The management of moderate to severe GO is challenging, requiring a multidisciplinary team of both endocrinologists and ophthalmologists. A survey of physicians who treat patients with GO published in 2006 reported that suboptimal management of these patients is widespread (5). A recently published consensus statement by the European Group on GO (EUGOGO) (6, 7), aiming to improve outcome of patients with GO, emphasized the need for an evidence-based approach in treating these patients.
To address this need, we performed a systematic review and metaanalysis of all randomized, controlled trials (RCTs) reporting therapeutic interventions for GO compared with placebo, no treatment, or other interventions.
Materials and Methods
Data source
We searched PubMed (January 1966 to June 2008), Cochrane Library (up to 2008, issue 3) for the term Graves ophthalmopathy or Graves orbitopathy and similar, crossed with specific treatment regimens and Cochrane highly sensitive search strategy for RCTs (Cochrane handbook for systematic reviews of interventions 5.0.1, http://www.cochrane.org/resources/handbook). We scanned references of all included trials and reviews identified for additional studies.
Study selection
We included all RCTs comparing treatment modalities for GO with placebo, no-intervention, or other treatment modalities. We included trials regardless of publication status and language. We did not include studies evaluating treatments for Graves’ disease in which ophthalmopathy was a secondary outcome and did not include studies evaluating modalities aimed at alleviating selective complications of GO such as diplopia or exophthalmos. Two reviewers (H.S.-K. and E.R. or M.S.) independently inspected the references identified by the search and applied inclusion criteria. For possibly relevant articles or in cases of disagreement between the two reviewers, we obtained and independently inspected the full article.
Data extraction and quality assessment
Two reviewers (H.S.-K. and E.R. or M.S.) independently extracted data from included trials. In case of any disagreement between the two reviewers, a third reviewer extracted the data. We contacted the authors for missing data when necessary. Two independent reviewers assessed trials for methodological quality. We individually assessed the following components: allocation concealment, generation of the allocation sequence, and blinding. We graded allocation concealment and generation as adequate, unclear, or inadequate (Cochrane handbook for systematic reviews of interventions 5.0.1, http://www.cochrane.org/resources/handbook). We also collected data on exclusions after randomization and whether the primary analysis was performed according to the intention-to-treat principle or per protocol.
Definition of outcomes
The primary outcome was the clinical activity score (CAS) at the end of follow-up, as defined in each study. CAS is a validated scoring system, designed to distinguish inflammatory from noninflammatory GO, and has a high predictive value for the outcome of immunosuppressive treatment in GO patients (8). It is based on the classical signs of inflammation: pain (2 points), redness (2 points), swelling (4 points), and impaired function (2 points). After two consecutive clinical examinations, an activity score can be determined, ranging from 0 to 10 points. Because determination of CAS requires two clinical examinations, a modification was proposed that allows determination of CAS in a single session (9). This modified CAS system includes seven items and will be referred to as the seven-item CAS. The two methods differ in evaluation of visual acuity, diplopia, and proptosis, which are part of the full 10-item CAS and are not included in the seven-item CAS. In studies performed before the introduction of the CAS scoring system, the ophthalmopathy index (OI) as proposed by Donaldson et al. (10) or the total eye score (TES) (11) were considered the primary outcome. Secondary outcomes included the NOSPECS scheme (mnemonic for no signs or symptoms, only signs, soft tissue involvement, proptosis, extraocular muscle involvement, corneal involvement and sight loss, graded as O, A, B, or C) (12); diplopia; proptosis; optic neuropathy in either eye; subjective outcome measures (e.g. cosmetic response satisfaction); visual acuity; and local eye irritation. Safety outcome was collected for all adverse events, serious adverse events leading to treatment discontinuation, and life-threatening events.
Comparisons
We prospectively defined the following comparisons: corticosteroids therapy vs. placebo or control, iv vs. oral corticosteroids, orbital irradiation vs. sham irradiation or control, corticosteroids vs. orbital irradiation, somatostatin analogs vs. placebo or control, combination of corticosteroids and orbital irradiation vs. either intervention alone, and comparison between various dosages of corticosteroids or orbital irradiation.
Data synthesis and analysis
We analyzed data by calculating the odds ratio (OR) for dichotomous variables and the mean difference or the standardized mean difference (SMD) for continuous variables, with uncertainty expressed with 95% confidence intervals (CI) [Review Manager (RevMan) computer program, version 5.0; the Nordic Cochrane Centre, the Cochrane Collaboration, 2008, Copenhagen, Denmark]. For the main outcome, CAS at the end of follow-up, we performed a modified intention-to-treat analysis in which we included all known outcomes, even if excluded from the trial’s original analysis. We used a fixed-effect model by using the Mantel-Haenszel method for pooling trial results throughout the review unless statistically significant heterogeneity was found, in which case, we chose a random-effects model by using the DerSimonian and Laird method. When available data were expressed using measures of variation other than sd and when sufficient data were available, we calculated the mean and sd according to the methods described in the Cochrane handbook for systematic reviews of interventions (Cochrane handbook for systematic reviews of interventions 5.0.1, http://www.cochrane.org/resources/handbook). We assessed heterogeneity of trial results by calculating a χ2 test of heterogeneity and the I2 measure of inconsistency. We predefined significant heterogeneity as a χ2 test P < 0.1 or an I2 measure greater than 50% (13). We performed sensitivity analyses to assess the effect of the quality of allocation concealment, allocation generation, and blinding on trial results.
Results
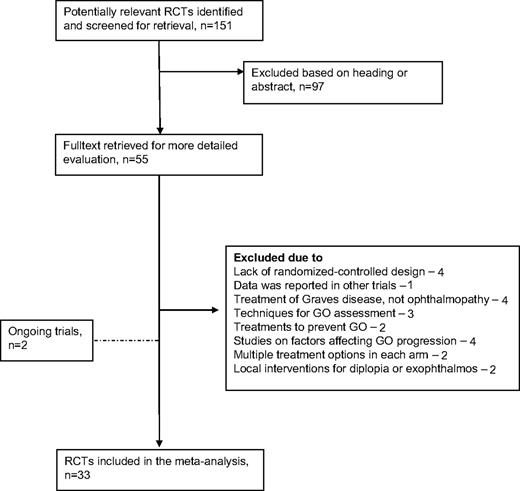
The literature search identified 55 trials of treatment modalities for GO. Reasons for exclusion are detailed in Fig. 1. Thirty-three trials published from 1983 to 2008, evaluating 1367 patients, fulfilled inclusion criteria (Table 1). The following comparisons were repeated in more than one trial: four trials compared oral corticosteroids with iv corticosteroids, four trials compared somatostatin analogs with placebo, three trials compared orbital irradiation with sham irradiation, three trials compared combination treatment of corticosteroids and orbital irradiation with either treatment alone, and two trials compared various dosages of orbital irradiation. Seventeen trials included comparisons that were not repeated in additional trials. Of these, four evaluated an intervention vs. placebo or control, and 13 included head-to-head comparisons. Patients in the included trials had moderately severe to severe GO (CAS > 5 or graded as moderately severe according to text) in 12 trials, moderate GO (mean or median CAS of 3–5 or according to text) in 18 trials, and mild or inactive GO (CAS < 3 or according to text) in three trials. Activity score at the end of follow-up was reported in 22 trials, of which it was defined as the primary outcome in nine trials, as part of a composite primary end point in eight trials, and as part of several outcome measures in five trials that did not define a primary end point. The 10-item CAS was used in eight trials, the seven-item CAS in eight trials, an undetermined type of CAS in one trial, the OI in three trials, the TES in one trial, and a self-designed activity score in one trial. In 19 trials patients were examined by the same ophthalmologist throughout the study, and color slides were used in four trials to assess soft tissue involvement. Patients with optic neuropathy were included in four trials. Seven trials were sponsored by pharmaceutical companies.
Included trials
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous vs. oral corticosteroids | ||||||||||||
| Aktaran et al. (14 ) | 2007 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 25 | 44.3 | CAS −5.2 (0.8) | N | N | A, A | Y | N |
| PO methylprednisolone (total 3.9 g) | 27 | 41.3 | CAS −5 (0.7) | |||||||||
| Kahaly et al. (15 ) | 2005 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 35 | Median 52 | CAS-Median 5 (3–7) | Y | N | B, B | Y | N |
| PO prednisolone (total 4.5–5 g) | 35 | Median 48 | ||||||||||
| Kauppinen-Makelin et al. (16 ) | 2002 | iv followed by PO methylprednisolone (total 4.16 g) | 14 wk | 12 months | 18 | 46.4 | CAS >3 or proptosis or diplopia | N | 13 radiation | A, A | N | N |
| PO prednisone (total 2990 g) | 16 wk | 15 | 46.1 | 11radiation | ||||||||
| Macchia et al. (17 ) | 2001 | iv methylprednisolone (total 12 g) | 6 wk | 2 yr | 25 | 42.6 | OI 4.43 (1.91) | N | N | B, B | Y | N |
| PO prednisone (60–80 mg/d, then taper) | 4–6 months | 26 | 44.57 | OI 2.65 (0.89) | ||||||||
| Somatostatin analogs vs. placebo | ||||||||||||
| Stan et al. (18 ) | 2006 | im octreotide LAR (20 mg/month) | 4 months | 4 months | 14 | Median 53 | CAS median 6.0 | N | 7 steroids | A, A | Y | N |
| Placebo | 11 | Median 61 | CAS median 5.0 | 3 steroids | ||||||||
| Chang and Liao (19 ) | 2006 | im lanreotide SR (60 mg/month) | 84 d | 84 d | 30 | 43 | CAS 3.6 (0.9) | N | N | A, A | Y | Y, Centapharm |
| Placebo | 30 | 43.1 | CAS 3.7 (0.8) | |||||||||
| Dickinson et al. (20 ) | 2004 | im octreotide LAR (30 mg/month) | 32 wk | 56 wk | 23 | Median 50 | CAS 5.39 (1.56) | N | 9 steroids, 5 radiation | A, A | Y | Y, Novartis |
| Placebo | 27 | CAS 5.85 (1.26) | 7 steroids, 4 radiation | |||||||||
| Wemeau et al. (21 ) | 2005 | im octreotide LAR (30 mg/month) | 4 months | 6 months | 26 | 47.5 | CAS 4.2 (1.61) | N | NS | B, A | Y | Y, Novartis |
| Placebo | 25 | 47.1 | CAS 4.5 (1.26) | |||||||||
| Orbital radiation (OR) vs. control | ||||||||||||
| Prummel et al. (22 ) | 2004 | OR 20 Gy | 2 wk | 12 months | 44 | 45.2 | CAS 3 (1.3) | N | NS | A, A | Y | N |
| Sham radiotherapy | 44 | 45.1 | CAS-3.3 (1.5) | |||||||||
| Gorman et al. (23 ) | 2001/2003 | OR 20 Gy | 12 d | 1 yr | 42 | Median 48 | Mild to moderate GO | N | 19 steroids | A, A | Y | N |
| Sham radiotherapy | ||||||||||||
| Mourits et al. (24 ) | 2000 | OR 20 Gy | 2 wk | 24 wk | 30 | 48.7 | CAS 3.3 (1.4) | N | N | A, A | Y | N |
| Sham radiotherapy | 30 | 49 | CAS 3.4 (1.3) | |||||||||
| Dosage of radiotherapy | ||||||||||||
| Gerling et al. (28 ) | 2003 | OR 2.4 Gy | 16 d | 6 months | 49 | Median 49 | Progressive GO | N | 5 steroids | A, A | Y | N |
| OR 16 Gy | 48 | 4 steroids | ||||||||||
| Kahaly et al. (29 ) | 2000 | OR 1 Gy/wk, total 20 Gy | 20 wk | 24 wk | 18 | Median 48 | CAS 5.5 | N | N | B, B | Y | N |
| OR 1 Gy/d, total 10 Gy | 2 wk | 22 | Median 47 | CAS 5 | ||||||||
| OR 2 Gy/d, total 20 Gy | 2 wk | 22 | Median 49 | CAS 5.5 | ||||||||
| Corticosteroids plus OR vs. either treatment alone | ||||||||||||
| Ng et al. (25 ) | 2005 | iv methylprednisolone (total 1500 mg), then PO prednisolone 0.7 mg/kg then taper | 3 months | 52 wk | 7 | 48.3 | TES 18.1 (8.7) | 2 | N | B, B | Y | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 3 months | 8 | 64.1 | TES 20.6 (8.5) | 3 | |||||||
| Bartalena et al. (26 ) | 1983 | PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 26 months | 12 | 46 | OI 6.2 | NS | N | A, B | N | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 5–6 months | 12 | 42 | OI 6.4 | ||||||||
| Marcocci et al. (27 ) | 1991 | OR 20 Gy PO prednisone 100 mg/d then taper | OR 2 wk, CS 5–6 months | 6–18 months | 13 | 47.3 | CAS 5.85 | NS | 3 steroids | A, A | Y | N |
| OR 20 Gy | 2 wk | 13 | 46 | CAS 5.46 | 2 steroids |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous vs. oral corticosteroids | ||||||||||||
| Aktaran et al. (14 ) | 2007 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 25 | 44.3 | CAS −5.2 (0.8) | N | N | A, A | Y | N |
| PO methylprednisolone (total 3.9 g) | 27 | 41.3 | CAS −5 (0.7) | |||||||||
| Kahaly et al. (15 ) | 2005 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 35 | Median 52 | CAS-Median 5 (3–7) | Y | N | B, B | Y | N |
| PO prednisolone (total 4.5–5 g) | 35 | Median 48 | ||||||||||
| Kauppinen-Makelin et al. (16 ) | 2002 | iv followed by PO methylprednisolone (total 4.16 g) | 14 wk | 12 months | 18 | 46.4 | CAS >3 or proptosis or diplopia | N | 13 radiation | A, A | N | N |
| PO prednisone (total 2990 g) | 16 wk | 15 | 46.1 | 11radiation | ||||||||
| Macchia et al. (17 ) | 2001 | iv methylprednisolone (total 12 g) | 6 wk | 2 yr | 25 | 42.6 | OI 4.43 (1.91) | N | N | B, B | Y | N |
| PO prednisone (60–80 mg/d, then taper) | 4–6 months | 26 | 44.57 | OI 2.65 (0.89) | ||||||||
| Somatostatin analogs vs. placebo | ||||||||||||
| Stan et al. (18 ) | 2006 | im octreotide LAR (20 mg/month) | 4 months | 4 months | 14 | Median 53 | CAS median 6.0 | N | 7 steroids | A, A | Y | N |
| Placebo | 11 | Median 61 | CAS median 5.0 | 3 steroids | ||||||||
| Chang and Liao (19 ) | 2006 | im lanreotide SR (60 mg/month) | 84 d | 84 d | 30 | 43 | CAS 3.6 (0.9) | N | N | A, A | Y | Y, Centapharm |
| Placebo | 30 | 43.1 | CAS 3.7 (0.8) | |||||||||
| Dickinson et al. (20 ) | 2004 | im octreotide LAR (30 mg/month) | 32 wk | 56 wk | 23 | Median 50 | CAS 5.39 (1.56) | N | 9 steroids, 5 radiation | A, A | Y | Y, Novartis |
| Placebo | 27 | CAS 5.85 (1.26) | 7 steroids, 4 radiation | |||||||||
| Wemeau et al. (21 ) | 2005 | im octreotide LAR (30 mg/month) | 4 months | 6 months | 26 | 47.5 | CAS 4.2 (1.61) | N | NS | B, A | Y | Y, Novartis |
| Placebo | 25 | 47.1 | CAS 4.5 (1.26) | |||||||||
| Orbital radiation (OR) vs. control | ||||||||||||
| Prummel et al. (22 ) | 2004 | OR 20 Gy | 2 wk | 12 months | 44 | 45.2 | CAS 3 (1.3) | N | NS | A, A | Y | N |
| Sham radiotherapy | 44 | 45.1 | CAS-3.3 (1.5) | |||||||||
| Gorman et al. (23 ) | 2001/2003 | OR 20 Gy | 12 d | 1 yr | 42 | Median 48 | Mild to moderate GO | N | 19 steroids | A, A | Y | N |
| Sham radiotherapy | ||||||||||||
| Mourits et al. (24 ) | 2000 | OR 20 Gy | 2 wk | 24 wk | 30 | 48.7 | CAS 3.3 (1.4) | N | N | A, A | Y | N |
| Sham radiotherapy | 30 | 49 | CAS 3.4 (1.3) | |||||||||
| Dosage of radiotherapy | ||||||||||||
| Gerling et al. (28 ) | 2003 | OR 2.4 Gy | 16 d | 6 months | 49 | Median 49 | Progressive GO | N | 5 steroids | A, A | Y | N |
| OR 16 Gy | 48 | 4 steroids | ||||||||||
| Kahaly et al. (29 ) | 2000 | OR 1 Gy/wk, total 20 Gy | 20 wk | 24 wk | 18 | Median 48 | CAS 5.5 | N | N | B, B | Y | N |
| OR 1 Gy/d, total 10 Gy | 2 wk | 22 | Median 47 | CAS 5 | ||||||||
| OR 2 Gy/d, total 20 Gy | 2 wk | 22 | Median 49 | CAS 5.5 | ||||||||
| Corticosteroids plus OR vs. either treatment alone | ||||||||||||
| Ng et al. (25 ) | 2005 | iv methylprednisolone (total 1500 mg), then PO prednisolone 0.7 mg/kg then taper | 3 months | 52 wk | 7 | 48.3 | TES 18.1 (8.7) | 2 | N | B, B | Y | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 3 months | 8 | 64.1 | TES 20.6 (8.5) | 3 | |||||||
| Bartalena et al. (26 ) | 1983 | PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 26 months | 12 | 46 | OI 6.2 | NS | N | A, B | N | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 5–6 months | 12 | 42 | OI 6.4 | ||||||||
| Marcocci et al. (27 ) | 1991 | OR 20 Gy PO prednisone 100 mg/d then taper | OR 2 wk, CS 5–6 months | 6–18 months | 13 | 47.3 | CAS 5.85 | NS | 3 steroids | A, A | Y | N |
| OR 20 Gy | 2 wk | 13 | 46 | CAS 5.46 | 2 steroids |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other comparisons | ||||||||||||
| Marcocci et al. (35 ) | 2001 | OR 20 Gy PO prednisone 100 mg, then taper | 22 wk | 12 months | 41 | 48 | CAS 4.2 (1.1) | 9 | 9 steroids | A, A | Y | N |
| OR 20 Gy iv methylprednisolone (total 9–12 g) | 14 wk | 41 | 50 | CAS 4.5 (sd 1.2) | 14 | |||||||
| van Geest et al. (30 ) | 2008 | iv methylprednisolone (total 6 g) | 3 months | 48 wk | 15 | NS | Moderate-severe GO | N | N | A, A | Y | N |
| Placebo | ||||||||||||
| Stamato et al. (43 ) | 2006 | Colchicine 1.5 mg/d for 1 month, then 1 mg/d for 2 months | 3 months | 3 months | 11 | 46.2 | CAS 4.68 (1.75) | N | 10 steroids | C, C | N | N |
| PO prednisone 0.75 mg/kg · d then taper | 11 | 38.9 | CAS 3.59 (1.53) | 7 steroids | ||||||||
| Wakelkamp et al. (38 ) | 2005 | 3-wall orbital decompression surgery | 26 wk | 6 | 52 | CAS 6 (0.8) | Y | 1 steroids | A, A | N | N | |
| iv methylprednisolone (total 6 g), then PO prednisone 40 mg, then taper | 20 wk | 9 | 52 | CAS 6 (0.5) | ||||||||
| Kahaly et al. (40 ) | 1996 | IVIG 1 g/kg 4 wk, then taper | 18 wk | 20 wk | 21 | 48 | Active GO | N | 11 steroids or radiation | B, B | Y | N |
| PO prednisolone 100 mg/kg 4 wk, then taper | 20 wk | 19 | 47 | 9 steroids or radiation | ||||||||
| Kung et al. (41 ) | 1996 | sc octreotide 1 mg/kg 4 wk, then taper | 3 months | 3 months | 8 | 38.2 | CAS 3 | N | NS | B, B | Y | Y, Sandoz |
| PO prednisone 1 mg/kg/d 4 wk, then taper | 10 | 45.2 | CAS 5 | |||||||||
| Prummel et al. (34 ) | 1993 | PO prednisone 60 mg/d, then taper + sham OR | 5 months | 24 wk | 28 | 47 | NOSPECS 2b-6a | N | N | C, B | Y | N |
| OR 20 Gy + placebo | 28 | 46.6 | ||||||||||
| Prummel et al. (11 ) | 1989 | PO prednisone 60 mg/d, then taper | 12 wk | 12 wk | 18 | 49 | TES 12.9 (6.2) | Y | N | B, B | N | N |
| PO cyclosporine 7.5 mg/kg · d | 18 | 52 | TES 11.5 (6.7) | |||||||||
| Kahaly et al. (39 ) | 1986 | PO cyclosporine 5–7.5 mg/kg · d, PO prednisone 50–100 mg/d, then taper | Prednisone 10 wk, cyclosporine 12 months | 12 months | 20 | 48.6 | NOSPECS 3–5 | NS | 10 steroids, 1 radiation | C, C | N | N |
| PO prednisone 50–100 mg/d, then taper | 10 wk | 20 | 44.9 | 8 steroids, 2 radiation | ||||||||
| Finamor et al. (32 ) | 2004 | PO pentoxifylline (total 216 g) | 6 months, crossover 6 months | 6 + 6 months | 9 | 41.5 | CAS ≤3 | N | 5 steroids | A, A | Y | N |
| Placebo | 9 | 40 | 4 steroids | |||||||||
| Antonelli et al. (45 ) | 1992 | OR 20 Gy IVIG 400 mg/kg · d given 24 times | 8 months | 6 months | 7 | 46.7 | CAS 7.1 (1.6) | N | N | B, B | Y | N |
| IVIG 400 mg/kg · d given 24 times | 7 | 45.7 | CAS 6.6 (2.4) | |||||||||
| Kahaly et al. (42 ) | 1990 | Ciamexone 300 mg/d, PO prednisolone 50 mg/d, then taper | 6 months | 6 months | 26 | 52 | NOSPECS 2–6 | NS | 21 steroids | B, B | Y | Y, Sandoz |
| PO prednisone 50 mg/d, then taper | 25 | 50 | 19 steroids | |||||||||
| Ebner et al. (31 ) | 2004 | Peribulbar triamcinolone injection 20 mg, four doses | 4 wk | 24 wk | 24 | 50.3 | New diplopia (<6 mo) | N | NS | B, B | N | N |
| Control | 17 | 36.1 | ||||||||||
| Jarhult et al. (44 ) | 2005 | Total thyroidectomy | 3 yr | 21 | Median 42 | Moderate GO | N | 6 steroids, 1 surgery | B, B | Y | N | |
| Subtotal thyroidectomy | 22 | Median 44 | 6 steroids | |||||||||
| Menconi et al. (37 ) | 2007 | Near-total thyroidectomy, iv methylprednisolone (total 6–10 g) | 12 wk | 1 yr | 27 | Median 37 | CAS 3.0 | N | N | B, B | Y | N |
| Near-total thyroidectomy, I 30 Mci steroids, the same | 27 | Median 39 | CAS 2.9 | |||||||||
| Marcocci et al. (37 ) | 1987 | OR 20 Gy, retrobulbar methylprednisolone 40 mg, 14 times | 9 months | 18–24 months | 30 | 54 | OI 5.8 | NS | N | B, B | N | N |
| OR 20 Gy, PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 30 | 44 | |||||||||
| Rogvi-Hansen et al. (33 ) | 1991 | Acupuncture, 10–12 sessions | 8 wk | 8 wk | 8 | NS | Exophthalmos | N | 1 steroid | B, B | Y | N |
| Control | 8 | 2 steroids, 1 radiation |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other comparisons | ||||||||||||
| Marcocci et al. (35 ) | 2001 | OR 20 Gy PO prednisone 100 mg, then taper | 22 wk | 12 months | 41 | 48 | CAS 4.2 (1.1) | 9 | 9 steroids | A, A | Y | N |
| OR 20 Gy iv methylprednisolone (total 9–12 g) | 14 wk | 41 | 50 | CAS 4.5 (sd 1.2) | 14 | |||||||
| van Geest et al. (30 ) | 2008 | iv methylprednisolone (total 6 g) | 3 months | 48 wk | 15 | NS | Moderate-severe GO | N | N | A, A | Y | N |
| Placebo | ||||||||||||
| Stamato et al. (43 ) | 2006 | Colchicine 1.5 mg/d for 1 month, then 1 mg/d for 2 months | 3 months | 3 months | 11 | 46.2 | CAS 4.68 (1.75) | N | 10 steroids | C, C | N | N |
| PO prednisone 0.75 mg/kg · d then taper | 11 | 38.9 | CAS 3.59 (1.53) | 7 steroids | ||||||||
| Wakelkamp et al. (38 ) | 2005 | 3-wall orbital decompression surgery | 26 wk | 6 | 52 | CAS 6 (0.8) | Y | 1 steroids | A, A | N | N | |
| iv methylprednisolone (total 6 g), then PO prednisone 40 mg, then taper | 20 wk | 9 | 52 | CAS 6 (0.5) | ||||||||
| Kahaly et al. (40 ) | 1996 | IVIG 1 g/kg 4 wk, then taper | 18 wk | 20 wk | 21 | 48 | Active GO | N | 11 steroids or radiation | B, B | Y | N |
| PO prednisolone 100 mg/kg 4 wk, then taper | 20 wk | 19 | 47 | 9 steroids or radiation | ||||||||
| Kung et al. (41 ) | 1996 | sc octreotide 1 mg/kg 4 wk, then taper | 3 months | 3 months | 8 | 38.2 | CAS 3 | N | NS | B, B | Y | Y, Sandoz |
| PO prednisone 1 mg/kg/d 4 wk, then taper | 10 | 45.2 | CAS 5 | |||||||||
| Prummel et al. (34 ) | 1993 | PO prednisone 60 mg/d, then taper + sham OR | 5 months | 24 wk | 28 | 47 | NOSPECS 2b-6a | N | N | C, B | Y | N |
| OR 20 Gy + placebo | 28 | 46.6 | ||||||||||
| Prummel et al. (11 ) | 1989 | PO prednisone 60 mg/d, then taper | 12 wk | 12 wk | 18 | 49 | TES 12.9 (6.2) | Y | N | B, B | N | N |
| PO cyclosporine 7.5 mg/kg · d | 18 | 52 | TES 11.5 (6.7) | |||||||||
| Kahaly et al. (39 ) | 1986 | PO cyclosporine 5–7.5 mg/kg · d, PO prednisone 50–100 mg/d, then taper | Prednisone 10 wk, cyclosporine 12 months | 12 months | 20 | 48.6 | NOSPECS 3–5 | NS | 10 steroids, 1 radiation | C, C | N | N |
| PO prednisone 50–100 mg/d, then taper | 10 wk | 20 | 44.9 | 8 steroids, 2 radiation | ||||||||
| Finamor et al. (32 ) | 2004 | PO pentoxifylline (total 216 g) | 6 months, crossover 6 months | 6 + 6 months | 9 | 41.5 | CAS ≤3 | N | 5 steroids | A, A | Y | N |
| Placebo | 9 | 40 | 4 steroids | |||||||||
| Antonelli et al. (45 ) | 1992 | OR 20 Gy IVIG 400 mg/kg · d given 24 times | 8 months | 6 months | 7 | 46.7 | CAS 7.1 (1.6) | N | N | B, B | Y | N |
| IVIG 400 mg/kg · d given 24 times | 7 | 45.7 | CAS 6.6 (2.4) | |||||||||
| Kahaly et al. (42 ) | 1990 | Ciamexone 300 mg/d, PO prednisolone 50 mg/d, then taper | 6 months | 6 months | 26 | 52 | NOSPECS 2–6 | NS | 21 steroids | B, B | Y | Y, Sandoz |
| PO prednisone 50 mg/d, then taper | 25 | 50 | 19 steroids | |||||||||
| Ebner et al. (31 ) | 2004 | Peribulbar triamcinolone injection 20 mg, four doses | 4 wk | 24 wk | 24 | 50.3 | New diplopia (<6 mo) | N | NS | B, B | N | N |
| Control | 17 | 36.1 | ||||||||||
| Jarhult et al. (44 ) | 2005 | Total thyroidectomy | 3 yr | 21 | Median 42 | Moderate GO | N | 6 steroids, 1 surgery | B, B | Y | N | |
| Subtotal thyroidectomy | 22 | Median 44 | 6 steroids | |||||||||
| Menconi et al. (37 ) | 2007 | Near-total thyroidectomy, iv methylprednisolone (total 6–10 g) | 12 wk | 1 yr | 27 | Median 37 | CAS 3.0 | N | N | B, B | Y | N |
| Near-total thyroidectomy, I 30 Mci steroids, the same | 27 | Median 39 | CAS 2.9 | |||||||||
| Marcocci et al. (37 ) | 1987 | OR 20 Gy, retrobulbar methylprednisolone 40 mg, 14 times | 9 months | 18–24 months | 30 | 54 | OI 5.8 | NS | N | B, B | N | N |
| OR 20 Gy, PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 30 | 44 | |||||||||
| Rogvi-Hansen et al. (33 ) | 1991 | Acupuncture, 10–12 sessions | 8 wk | 8 wk | 8 | NS | Exophthalmos | N | 1 steroid | B, B | Y | N |
| Control | 8 | 2 steroids, 1 radiation |
Numbers given as mean (sd) unless otherwise specified. NS, Not specified; A, adequate; B, unknown; C, inadequate; Y, yes; N, no; PO, per os; CS, corticosteroids; Gy, Grey; MCi, millicurie.
Included trials
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous vs. oral corticosteroids | ||||||||||||
| Aktaran et al. (14 ) | 2007 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 25 | 44.3 | CAS −5.2 (0.8) | N | N | A, A | Y | N |
| PO methylprednisolone (total 3.9 g) | 27 | 41.3 | CAS −5 (0.7) | |||||||||
| Kahaly et al. (15 ) | 2005 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 35 | Median 52 | CAS-Median 5 (3–7) | Y | N | B, B | Y | N |
| PO prednisolone (total 4.5–5 g) | 35 | Median 48 | ||||||||||
| Kauppinen-Makelin et al. (16 ) | 2002 | iv followed by PO methylprednisolone (total 4.16 g) | 14 wk | 12 months | 18 | 46.4 | CAS >3 or proptosis or diplopia | N | 13 radiation | A, A | N | N |
| PO prednisone (total 2990 g) | 16 wk | 15 | 46.1 | 11radiation | ||||||||
| Macchia et al. (17 ) | 2001 | iv methylprednisolone (total 12 g) | 6 wk | 2 yr | 25 | 42.6 | OI 4.43 (1.91) | N | N | B, B | Y | N |
| PO prednisone (60–80 mg/d, then taper) | 4–6 months | 26 | 44.57 | OI 2.65 (0.89) | ||||||||
| Somatostatin analogs vs. placebo | ||||||||||||
| Stan et al. (18 ) | 2006 | im octreotide LAR (20 mg/month) | 4 months | 4 months | 14 | Median 53 | CAS median 6.0 | N | 7 steroids | A, A | Y | N |
| Placebo | 11 | Median 61 | CAS median 5.0 | 3 steroids | ||||||||
| Chang and Liao (19 ) | 2006 | im lanreotide SR (60 mg/month) | 84 d | 84 d | 30 | 43 | CAS 3.6 (0.9) | N | N | A, A | Y | Y, Centapharm |
| Placebo | 30 | 43.1 | CAS 3.7 (0.8) | |||||||||
| Dickinson et al. (20 ) | 2004 | im octreotide LAR (30 mg/month) | 32 wk | 56 wk | 23 | Median 50 | CAS 5.39 (1.56) | N | 9 steroids, 5 radiation | A, A | Y | Y, Novartis |
| Placebo | 27 | CAS 5.85 (1.26) | 7 steroids, 4 radiation | |||||||||
| Wemeau et al. (21 ) | 2005 | im octreotide LAR (30 mg/month) | 4 months | 6 months | 26 | 47.5 | CAS 4.2 (1.61) | N | NS | B, A | Y | Y, Novartis |
| Placebo | 25 | 47.1 | CAS 4.5 (1.26) | |||||||||
| Orbital radiation (OR) vs. control | ||||||||||||
| Prummel et al. (22 ) | 2004 | OR 20 Gy | 2 wk | 12 months | 44 | 45.2 | CAS 3 (1.3) | N | NS | A, A | Y | N |
| Sham radiotherapy | 44 | 45.1 | CAS-3.3 (1.5) | |||||||||
| Gorman et al. (23 ) | 2001/2003 | OR 20 Gy | 12 d | 1 yr | 42 | Median 48 | Mild to moderate GO | N | 19 steroids | A, A | Y | N |
| Sham radiotherapy | ||||||||||||
| Mourits et al. (24 ) | 2000 | OR 20 Gy | 2 wk | 24 wk | 30 | 48.7 | CAS 3.3 (1.4) | N | N | A, A | Y | N |
| Sham radiotherapy | 30 | 49 | CAS 3.4 (1.3) | |||||||||
| Dosage of radiotherapy | ||||||||||||
| Gerling et al. (28 ) | 2003 | OR 2.4 Gy | 16 d | 6 months | 49 | Median 49 | Progressive GO | N | 5 steroids | A, A | Y | N |
| OR 16 Gy | 48 | 4 steroids | ||||||||||
| Kahaly et al. (29 ) | 2000 | OR 1 Gy/wk, total 20 Gy | 20 wk | 24 wk | 18 | Median 48 | CAS 5.5 | N | N | B, B | Y | N |
| OR 1 Gy/d, total 10 Gy | 2 wk | 22 | Median 47 | CAS 5 | ||||||||
| OR 2 Gy/d, total 20 Gy | 2 wk | 22 | Median 49 | CAS 5.5 | ||||||||
| Corticosteroids plus OR vs. either treatment alone | ||||||||||||
| Ng et al. (25 ) | 2005 | iv methylprednisolone (total 1500 mg), then PO prednisolone 0.7 mg/kg then taper | 3 months | 52 wk | 7 | 48.3 | TES 18.1 (8.7) | 2 | N | B, B | Y | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 3 months | 8 | 64.1 | TES 20.6 (8.5) | 3 | |||||||
| Bartalena et al. (26 ) | 1983 | PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 26 months | 12 | 46 | OI 6.2 | NS | N | A, B | N | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 5–6 months | 12 | 42 | OI 6.4 | ||||||||
| Marcocci et al. (27 ) | 1991 | OR 20 Gy PO prednisone 100 mg/d then taper | OR 2 wk, CS 5–6 months | 6–18 months | 13 | 47.3 | CAS 5.85 | NS | 3 steroids | A, A | Y | N |
| OR 20 Gy | 2 wk | 13 | 46 | CAS 5.46 | 2 steroids |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intravenous vs. oral corticosteroids | ||||||||||||
| Aktaran et al. (14 ) | 2007 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 25 | 44.3 | CAS −5.2 (0.8) | N | N | A, A | Y | N |
| PO methylprednisolone (total 3.9 g) | 27 | 41.3 | CAS −5 (0.7) | |||||||||
| Kahaly et al. (15 ) | 2005 | iv methylprednisolone (total 4.5 g) | 12 wk | 12 wk | 35 | Median 52 | CAS-Median 5 (3–7) | Y | N | B, B | Y | N |
| PO prednisolone (total 4.5–5 g) | 35 | Median 48 | ||||||||||
| Kauppinen-Makelin et al. (16 ) | 2002 | iv followed by PO methylprednisolone (total 4.16 g) | 14 wk | 12 months | 18 | 46.4 | CAS >3 or proptosis or diplopia | N | 13 radiation | A, A | N | N |
| PO prednisone (total 2990 g) | 16 wk | 15 | 46.1 | 11radiation | ||||||||
| Macchia et al. (17 ) | 2001 | iv methylprednisolone (total 12 g) | 6 wk | 2 yr | 25 | 42.6 | OI 4.43 (1.91) | N | N | B, B | Y | N |
| PO prednisone (60–80 mg/d, then taper) | 4–6 months | 26 | 44.57 | OI 2.65 (0.89) | ||||||||
| Somatostatin analogs vs. placebo | ||||||||||||
| Stan et al. (18 ) | 2006 | im octreotide LAR (20 mg/month) | 4 months | 4 months | 14 | Median 53 | CAS median 6.0 | N | 7 steroids | A, A | Y | N |
| Placebo | 11 | Median 61 | CAS median 5.0 | 3 steroids | ||||||||
| Chang and Liao (19 ) | 2006 | im lanreotide SR (60 mg/month) | 84 d | 84 d | 30 | 43 | CAS 3.6 (0.9) | N | N | A, A | Y | Y, Centapharm |
| Placebo | 30 | 43.1 | CAS 3.7 (0.8) | |||||||||
| Dickinson et al. (20 ) | 2004 | im octreotide LAR (30 mg/month) | 32 wk | 56 wk | 23 | Median 50 | CAS 5.39 (1.56) | N | 9 steroids, 5 radiation | A, A | Y | Y, Novartis |
| Placebo | 27 | CAS 5.85 (1.26) | 7 steroids, 4 radiation | |||||||||
| Wemeau et al. (21 ) | 2005 | im octreotide LAR (30 mg/month) | 4 months | 6 months | 26 | 47.5 | CAS 4.2 (1.61) | N | NS | B, A | Y | Y, Novartis |
| Placebo | 25 | 47.1 | CAS 4.5 (1.26) | |||||||||
| Orbital radiation (OR) vs. control | ||||||||||||
| Prummel et al. (22 ) | 2004 | OR 20 Gy | 2 wk | 12 months | 44 | 45.2 | CAS 3 (1.3) | N | NS | A, A | Y | N |
| Sham radiotherapy | 44 | 45.1 | CAS-3.3 (1.5) | |||||||||
| Gorman et al. (23 ) | 2001/2003 | OR 20 Gy | 12 d | 1 yr | 42 | Median 48 | Mild to moderate GO | N | 19 steroids | A, A | Y | N |
| Sham radiotherapy | ||||||||||||
| Mourits et al. (24 ) | 2000 | OR 20 Gy | 2 wk | 24 wk | 30 | 48.7 | CAS 3.3 (1.4) | N | N | A, A | Y | N |
| Sham radiotherapy | 30 | 49 | CAS 3.4 (1.3) | |||||||||
| Dosage of radiotherapy | ||||||||||||
| Gerling et al. (28 ) | 2003 | OR 2.4 Gy | 16 d | 6 months | 49 | Median 49 | Progressive GO | N | 5 steroids | A, A | Y | N |
| OR 16 Gy | 48 | 4 steroids | ||||||||||
| Kahaly et al. (29 ) | 2000 | OR 1 Gy/wk, total 20 Gy | 20 wk | 24 wk | 18 | Median 48 | CAS 5.5 | N | N | B, B | Y | N |
| OR 1 Gy/d, total 10 Gy | 2 wk | 22 | Median 47 | CAS 5 | ||||||||
| OR 2 Gy/d, total 20 Gy | 2 wk | 22 | Median 49 | CAS 5.5 | ||||||||
| Corticosteroids plus OR vs. either treatment alone | ||||||||||||
| Ng et al. (25 ) | 2005 | iv methylprednisolone (total 1500 mg), then PO prednisolone 0.7 mg/kg then taper | 3 months | 52 wk | 7 | 48.3 | TES 18.1 (8.7) | 2 | N | B, B | Y | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 3 months | 8 | 64.1 | TES 20.6 (8.5) | 3 | |||||||
| Bartalena et al. (26 ) | 1983 | PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 26 months | 12 | 46 | OI 6.2 | NS | N | A, B | N | N |
| OR 20 Gy steroids, same protocol | OR 2 wk, CS 5–6 months | 12 | 42 | OI 6.4 | ||||||||
| Marcocci et al. (27 ) | 1991 | OR 20 Gy PO prednisone 100 mg/d then taper | OR 2 wk, CS 5–6 months | 6–18 months | 13 | 47.3 | CAS 5.85 | NS | 3 steroids | A, A | Y | N |
| OR 20 Gy | 2 wk | 13 | 46 | CAS 5.46 | 2 steroids |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other comparisons | ||||||||||||
| Marcocci et al. (35 ) | 2001 | OR 20 Gy PO prednisone 100 mg, then taper | 22 wk | 12 months | 41 | 48 | CAS 4.2 (1.1) | 9 | 9 steroids | A, A | Y | N |
| OR 20 Gy iv methylprednisolone (total 9–12 g) | 14 wk | 41 | 50 | CAS 4.5 (sd 1.2) | 14 | |||||||
| van Geest et al. (30 ) | 2008 | iv methylprednisolone (total 6 g) | 3 months | 48 wk | 15 | NS | Moderate-severe GO | N | N | A, A | Y | N |
| Placebo | ||||||||||||
| Stamato et al. (43 ) | 2006 | Colchicine 1.5 mg/d for 1 month, then 1 mg/d for 2 months | 3 months | 3 months | 11 | 46.2 | CAS 4.68 (1.75) | N | 10 steroids | C, C | N | N |
| PO prednisone 0.75 mg/kg · d then taper | 11 | 38.9 | CAS 3.59 (1.53) | 7 steroids | ||||||||
| Wakelkamp et al. (38 ) | 2005 | 3-wall orbital decompression surgery | 26 wk | 6 | 52 | CAS 6 (0.8) | Y | 1 steroids | A, A | N | N | |
| iv methylprednisolone (total 6 g), then PO prednisone 40 mg, then taper | 20 wk | 9 | 52 | CAS 6 (0.5) | ||||||||
| Kahaly et al. (40 ) | 1996 | IVIG 1 g/kg 4 wk, then taper | 18 wk | 20 wk | 21 | 48 | Active GO | N | 11 steroids or radiation | B, B | Y | N |
| PO prednisolone 100 mg/kg 4 wk, then taper | 20 wk | 19 | 47 | 9 steroids or radiation | ||||||||
| Kung et al. (41 ) | 1996 | sc octreotide 1 mg/kg 4 wk, then taper | 3 months | 3 months | 8 | 38.2 | CAS 3 | N | NS | B, B | Y | Y, Sandoz |
| PO prednisone 1 mg/kg/d 4 wk, then taper | 10 | 45.2 | CAS 5 | |||||||||
| Prummel et al. (34 ) | 1993 | PO prednisone 60 mg/d, then taper + sham OR | 5 months | 24 wk | 28 | 47 | NOSPECS 2b-6a | N | N | C, B | Y | N |
| OR 20 Gy + placebo | 28 | 46.6 | ||||||||||
| Prummel et al. (11 ) | 1989 | PO prednisone 60 mg/d, then taper | 12 wk | 12 wk | 18 | 49 | TES 12.9 (6.2) | Y | N | B, B | N | N |
| PO cyclosporine 7.5 mg/kg · d | 18 | 52 | TES 11.5 (6.7) | |||||||||
| Kahaly et al. (39 ) | 1986 | PO cyclosporine 5–7.5 mg/kg · d, PO prednisone 50–100 mg/d, then taper | Prednisone 10 wk, cyclosporine 12 months | 12 months | 20 | 48.6 | NOSPECS 3–5 | NS | 10 steroids, 1 radiation | C, C | N | N |
| PO prednisone 50–100 mg/d, then taper | 10 wk | 20 | 44.9 | 8 steroids, 2 radiation | ||||||||
| Finamor et al. (32 ) | 2004 | PO pentoxifylline (total 216 g) | 6 months, crossover 6 months | 6 + 6 months | 9 | 41.5 | CAS ≤3 | N | 5 steroids | A, A | Y | N |
| Placebo | 9 | 40 | 4 steroids | |||||||||
| Antonelli et al. (45 ) | 1992 | OR 20 Gy IVIG 400 mg/kg · d given 24 times | 8 months | 6 months | 7 | 46.7 | CAS 7.1 (1.6) | N | N | B, B | Y | N |
| IVIG 400 mg/kg · d given 24 times | 7 | 45.7 | CAS 6.6 (2.4) | |||||||||
| Kahaly et al. (42 ) | 1990 | Ciamexone 300 mg/d, PO prednisolone 50 mg/d, then taper | 6 months | 6 months | 26 | 52 | NOSPECS 2–6 | NS | 21 steroids | B, B | Y | Y, Sandoz |
| PO prednisone 50 mg/d, then taper | 25 | 50 | 19 steroids | |||||||||
| Ebner et al. (31 ) | 2004 | Peribulbar triamcinolone injection 20 mg, four doses | 4 wk | 24 wk | 24 | 50.3 | New diplopia (<6 mo) | N | NS | B, B | N | N |
| Control | 17 | 36.1 | ||||||||||
| Jarhult et al. (44 ) | 2005 | Total thyroidectomy | 3 yr | 21 | Median 42 | Moderate GO | N | 6 steroids, 1 surgery | B, B | Y | N | |
| Subtotal thyroidectomy | 22 | Median 44 | 6 steroids | |||||||||
| Menconi et al. (37 ) | 2007 | Near-total thyroidectomy, iv methylprednisolone (total 6–10 g) | 12 wk | 1 yr | 27 | Median 37 | CAS 3.0 | N | N | B, B | Y | N |
| Near-total thyroidectomy, I 30 Mci steroids, the same | 27 | Median 39 | CAS 2.9 | |||||||||
| Marcocci et al. (37 ) | 1987 | OR 20 Gy, retrobulbar methylprednisolone 40 mg, 14 times | 9 months | 18–24 months | 30 | 54 | OI 5.8 | NS | N | B, B | N | N |
| OR 20 Gy, PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 30 | 44 | |||||||||
| Rogvi-Hansen et al. (33 ) | 1991 | Acupuncture, 10–12 sessions | 8 wk | 8 wk | 8 | NS | Exophthalmos | N | 1 steroid | B, B | Y | N |
| Control | 8 | 2 steroids, 1 radiation |
| Study . | Year . | Treatment (dose) . | Treatment duration . | Follow-up . | Patients (n) . | Mean age (yr) . | GO severity (mean, sd) . | Optic neuropathy . | Prior treatment . | Allocation generation, concealment . | Binding . | Sponsored y/n . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Other comparisons | ||||||||||||
| Marcocci et al. (35 ) | 2001 | OR 20 Gy PO prednisone 100 mg, then taper | 22 wk | 12 months | 41 | 48 | CAS 4.2 (1.1) | 9 | 9 steroids | A, A | Y | N |
| OR 20 Gy iv methylprednisolone (total 9–12 g) | 14 wk | 41 | 50 | CAS 4.5 (sd 1.2) | 14 | |||||||
| van Geest et al. (30 ) | 2008 | iv methylprednisolone (total 6 g) | 3 months | 48 wk | 15 | NS | Moderate-severe GO | N | N | A, A | Y | N |
| Placebo | ||||||||||||
| Stamato et al. (43 ) | 2006 | Colchicine 1.5 mg/d for 1 month, then 1 mg/d for 2 months | 3 months | 3 months | 11 | 46.2 | CAS 4.68 (1.75) | N | 10 steroids | C, C | N | N |
| PO prednisone 0.75 mg/kg · d then taper | 11 | 38.9 | CAS 3.59 (1.53) | 7 steroids | ||||||||
| Wakelkamp et al. (38 ) | 2005 | 3-wall orbital decompression surgery | 26 wk | 6 | 52 | CAS 6 (0.8) | Y | 1 steroids | A, A | N | N | |
| iv methylprednisolone (total 6 g), then PO prednisone 40 mg, then taper | 20 wk | 9 | 52 | CAS 6 (0.5) | ||||||||
| Kahaly et al. (40 ) | 1996 | IVIG 1 g/kg 4 wk, then taper | 18 wk | 20 wk | 21 | 48 | Active GO | N | 11 steroids or radiation | B, B | Y | N |
| PO prednisolone 100 mg/kg 4 wk, then taper | 20 wk | 19 | 47 | 9 steroids or radiation | ||||||||
| Kung et al. (41 ) | 1996 | sc octreotide 1 mg/kg 4 wk, then taper | 3 months | 3 months | 8 | 38.2 | CAS 3 | N | NS | B, B | Y | Y, Sandoz |
| PO prednisone 1 mg/kg/d 4 wk, then taper | 10 | 45.2 | CAS 5 | |||||||||
| Prummel et al. (34 ) | 1993 | PO prednisone 60 mg/d, then taper + sham OR | 5 months | 24 wk | 28 | 47 | NOSPECS 2b-6a | N | N | C, B | Y | N |
| OR 20 Gy + placebo | 28 | 46.6 | ||||||||||
| Prummel et al. (11 ) | 1989 | PO prednisone 60 mg/d, then taper | 12 wk | 12 wk | 18 | 49 | TES 12.9 (6.2) | Y | N | B, B | N | N |
| PO cyclosporine 7.5 mg/kg · d | 18 | 52 | TES 11.5 (6.7) | |||||||||
| Kahaly et al. (39 ) | 1986 | PO cyclosporine 5–7.5 mg/kg · d, PO prednisone 50–100 mg/d, then taper | Prednisone 10 wk, cyclosporine 12 months | 12 months | 20 | 48.6 | NOSPECS 3–5 | NS | 10 steroids, 1 radiation | C, C | N | N |
| PO prednisone 50–100 mg/d, then taper | 10 wk | 20 | 44.9 | 8 steroids, 2 radiation | ||||||||
| Finamor et al. (32 ) | 2004 | PO pentoxifylline (total 216 g) | 6 months, crossover 6 months | 6 + 6 months | 9 | 41.5 | CAS ≤3 | N | 5 steroids | A, A | Y | N |
| Placebo | 9 | 40 | 4 steroids | |||||||||
| Antonelli et al. (45 ) | 1992 | OR 20 Gy IVIG 400 mg/kg · d given 24 times | 8 months | 6 months | 7 | 46.7 | CAS 7.1 (1.6) | N | N | B, B | Y | N |
| IVIG 400 mg/kg · d given 24 times | 7 | 45.7 | CAS 6.6 (2.4) | |||||||||
| Kahaly et al. (42 ) | 1990 | Ciamexone 300 mg/d, PO prednisolone 50 mg/d, then taper | 6 months | 6 months | 26 | 52 | NOSPECS 2–6 | NS | 21 steroids | B, B | Y | Y, Sandoz |
| PO prednisone 50 mg/d, then taper | 25 | 50 | 19 steroids | |||||||||
| Ebner et al. (31 ) | 2004 | Peribulbar triamcinolone injection 20 mg, four doses | 4 wk | 24 wk | 24 | 50.3 | New diplopia (<6 mo) | N | NS | B, B | N | N |
| Control | 17 | 36.1 | ||||||||||
| Jarhult et al. (44 ) | 2005 | Total thyroidectomy | 3 yr | 21 | Median 42 | Moderate GO | N | 6 steroids, 1 surgery | B, B | Y | N | |
| Subtotal thyroidectomy | 22 | Median 44 | 6 steroids | |||||||||
| Menconi et al. (37 ) | 2007 | Near-total thyroidectomy, iv methylprednisolone (total 6–10 g) | 12 wk | 1 yr | 27 | Median 37 | CAS 3.0 | N | N | B, B | Y | N |
| Near-total thyroidectomy, I 30 Mci steroids, the same | 27 | Median 39 | CAS 2.9 | |||||||||
| Marcocci et al. (37 ) | 1987 | OR 20 Gy, retrobulbar methylprednisolone 40 mg, 14 times | 9 months | 18–24 months | 30 | 54 | OI 5.8 | NS | N | B, B | N | N |
| OR 20 Gy, PO methylprednisolone 70–80 mg/d, then taper | 5–6 months | 30 | 44 | |||||||||
| Rogvi-Hansen et al. (33 ) | 1991 | Acupuncture, 10–12 sessions | 8 wk | 8 wk | 8 | NS | Exophthalmos | N | 1 steroid | B, B | Y | N |
| Control | 8 | 2 steroids, 1 radiation |
Numbers given as mean (sd) unless otherwise specified. NS, Not specified; A, adequate; B, unknown; C, inadequate; Y, yes; N, no; PO, per os; CS, corticosteroids; Gy, Grey; MCi, millicurie.
Oral corticosteroids vs. iv corticosteroids
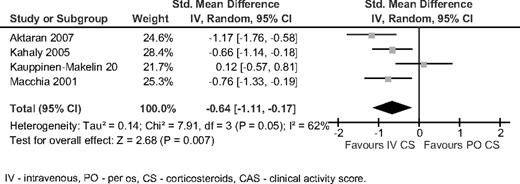
Four trials compared oral with iv corticosteroids (14–17). Dosing regimens are detailed in Table 1. Duration of treatment ranged from 6 to 16 wk for iv corticosteroids and from 12 to 24 wk for oral regimens. Intravenous corticosteroids were significantly better than oral corticosteroids in reducing CAS at the end of follow-up (SMD −0.64, 95% CI −1.11 to −0.17, χ2 7.91, I2 62%, random effect) (Fig. 2). This advantage was mostly due to the results in patients with severe GO (baseline CAS > 5) (SMD −0.86, 95% CI −1.24 to −0.49). There was no difference in proptosis, diplopia, lid aperture, and visual acuity between the groups. In patients receiving oral corticosteroids, there were significantly more adverse events than in the iv group (OR 0.12, 95% CI 0.05–0.26). Patients in the oral group had high rate of steroid-related adverse events, mostly weight gain (26%), hypertension (8%), and cushingoid features (7%). Adverse events in the iv group included lower rates of steroid-related events (3–4%) and common mild symptoms occurring during infusion or within 24 h of treatment consisting of palpitations (8%), flushes (20%), and transient dyspepsia (15%). Treatment was discontinued in four patients in the oral group and none of the iv group.
Intravenous corticosteroids vs. oral corticosteroids. The outcome was CAS at the end of follow-up. PO, Per os; CS, corticosteroids.
Somatostatin analogs vs. placebo
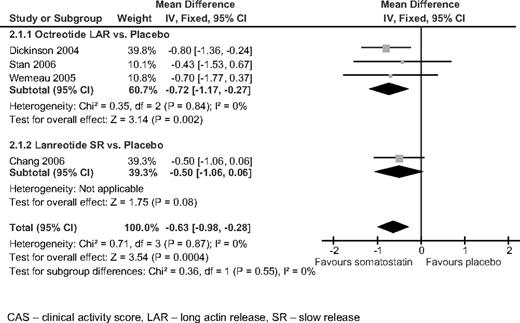
Four trials compared long-acting release (LAR) formulations of somatostatin analogs [octreotide LAR and lanreotide slow release (SR)] with placebo (18–21). The treatment duration ranged from 3 to 8 months, with follow-up periods of 3–14 months. Three trials were sponsored by pharmaceutical companies. Included patients had active GO, with CAS score of 3 or more. The combined results at the end of follow-up showed a minor but statistically significant lower CAS for patients treated with somatostatin analogs over placebo (mean difference −0.63, 95% CI −0.98 to −0.28) (Fig. 3). There was no advantage for somatostatin analogs in other outcomes, including diplopia, proptosis, and lid aperture. Patients in the somatostatin analog group had significantly more adverse events (OR 2.57, 95% CI 1.09–6.05), mostly gastrointestinal (in 60% of patients), that did not result in discontinuation of the drug.
Somatostatin analogs vs. placebo. The outcome was CAS at the end of follow-up.
Orbital radiotherapy vs. control
Three trials compared orbital radiotherapy with control (sham radiotherapy) (22–24). Retrobulbar radiotherapy was administered in 10 divided fractions of 2 Gy (total 20 Gy) in all studies. Follow-up period ranged from 24 wk to 1 yr. There was no advantage of radiotherapy over control in CAS, proptosis, or lid aperture outcomes. Radiotherapy was superior to control in response rate of diplopia (OR 4.88, 95% CI 1.93–12.34, two trials). Adverse events reported during the follow-up period were uncommon and mild and did not lead to discontinuation of radiation courses.
Orbital radiotherapy plus corticosteroids vs. radiation or corticosteroids alone
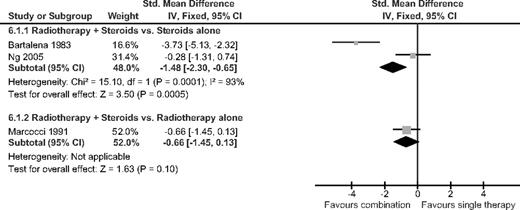
Combination treatment of systemic corticosteroids and radiotherapy was compared with steroid treatment alone in two trials (25, 26) and with radiotherapy alone in one trial (27). The corticosteroids were given orally and in one trial (25) were given iv for 3 d, followed by 3 months of oral therapy (Table 1). The response to treatment was evaluated using the OI and TES scoring systems. Treatment with combination of orbital radiotherapy and corticosteroids was significantly better than with either treatment alone (SMD −1.05, 95% CI −1.62 to −0.48) (Fig. 4). In the subgroup of orbital radiotherapy plus corticosteroids vs. corticosteroids alone, the addition of radiotherapy resulted in significantly better results (SMD −1.4, 95% CI −2.3 to −0.65). There was no difference between groups in proptosis and visual acuity at the end of follow-up.
Orbital radiotherapy plus corticosteroids vs. either treatment alone. The outcome was OI/TES at the end of follow-up.
Dosage of radiation
Two studies compared high- vs. low-dose radiation. The first (28) compared 16 with 2.4 Gy, and the other (29) compared 20 Gy (in two administration methods) with 10 Gy. The primary outcome was a composite end point, including the results of ophthalmologic and magnetic resonance imaging evaluation before and 6 months after treatment. In both trials there was no significant difference in efficacy between low- and high-dose radiation. However, lack of sufficient outcome data precluded the ability to combine the results for metaanalysis.
Other comparisons
Seventeen trials compared interventions for GO that were not repeated in additional trials. Four trials evaluated therapeutic interventions vs. placebo or control, and 13 evaluated head-to-head comparisons. Of these 17 trials, 13 included therapy with corticosteroids at least in one arm.
Treatment vs. placebo or control
Methylprednisolone pulse therapy resulted in a significant reduction of CAS compared with placebo (mean difference −1.17, 95% CI −2.15 to −0.18) (30); periocular injection of triamcinolone was effective in reducing diplopia when compared with control (31); pentoxifylline improved quality of life and proptosis compared with placebo in patients with inactive GO (32); and no improvement was found when acupuncture was compared with control (33).
Head-to-head comparisons
Comparison of oral corticosteroids with orbital radiotherapy in patients with moderately severe GO showed a similar response rate with both treatments (using the NOSPECS system), with more adverse events in the corticosteroid group (34). The combination of orbital radiotherapy with corticosteroids was evaluated in two trials: the first (35) evaluated orbital radiotherapy in combination with either oral or iv corticosteroids. In the iv corticosteroids plus radiotherapy group, the CAS score was significantly lower than in the oral corticosteroids plus radiotherapy group (mean difference 0.5, 95% CI 0.02–0.98), with a lower rate of adverse events. The second trial (36) compared oral corticosteroids plus orbital radiotherapy with retrobulbar corticosteroids injection plus orbital radiotherapy. Treatment with oral corticosteroids yielded better response rates and a greater reduction in the OI (mean decrease 3.5 vs. 2.6, P < 0.02) than the retrobulbar route.
The effect of iv corticosteroids with either near-total thyroidectomy or thyroidectomy plus I131 ablation (total thyroid ablation) was evaluated in one trial (37), with short-term advantage to the total thyroid ablation group.
In patients with active GO and optic neuropathy, surgical decompression was compared with iv corticosteroids as first-line treatment (38). The primary outcome was change in visual acuity. Surgery did not result in a better outcome, and in this group 83% of patients eventually needed additional iv corticosteroids therapy, whereas 56% of patients in the iv corticosteroids group needed additional therapy (surgery or orbital radiotherapy).
Two trials evaluated the effect of immunomodulation with cyclosporine in patients with active GO. The first evaluated cyclosporine in addition to oral corticosteroids vs. oral corticosteroids alone (39). In the combination group, there was a greater reduction in an activity score (which was defined within the manuscript) at 10 wk (mean 11.8 vs. 14.07, P < 0.05) and a lower relapse rate after corticosteroid discontinuation (5 vs. 40%). The second compared cyclosporine with oral corticosteroids (12), showing better response rate with oral corticosteroids as a single therapy.
Other interventions that were compared with corticosteroids include iv immunoglobulins (IVIGs), the somatostatin analog octreotide, the immunomodulator ciamexone combined with oral corticosteroids, and colchicine. The use of IVIG resulted in outcomes comparable with oral corticosteroids (40); octreotide had a similar overall efficacy as oral corticosteroids but was not as effective in reducing extraocular muscle size (41); the addition of ciamexone to oral corticosteroids as compared with oral corticosteroids alone did not show any effect on the course and activity of GO (42); colchicine had similar response rate as oral corticosteroids, with lower rate of adverse events (43).
Two additional trials compared interventions that did not include corticosteroids on either arm. Subtotal thyroidectomy in patients with active GO had a comparable beneficial effect as total thyroidectomy but with lower risk of surgical complications (44); the combination of IVIG with orbital radiotherapy had similar efficacy as treatment with IVIG alone (45).
Discussion
To guide optimal management of patients with GO, we performed a systematic review and metaanalysis of 33 RCTs evaluating 1367 patients. The most studied drugs were corticosteroids in various administration routes, included in 60% of trials. Intravenous pulse corticosteroids had a statistically significant advantage over oral corticosteroids for the primary outcome of clinical activity score, with a mean difference of 0.64 points at the end of follow-up. This advantage was also demonstrated when orbital radiotherapy was added to both arms. It is interesting to note that no RCT compared oral corticosteroids with placebo, and only one trial compared iv corticosteroids with placebo, documenting its efficacy. The adverse event profile, which is of major importance with corticosteroids therapy, clearly favored iv pulse therapy over oral therapy, with significantly lower rates of steroid-related events and lower rates of treatment discontinuation. Our findings support the consensus statement of the EUGOGO published in 2008 (6, 7) recommending iv corticosteroids as the treatment of choice for moderate to severe and active (CAS ≥3) GO. However, this treatment has several limitations, including cost (46), the need for a specialized treatment setting (hospital, outpatient clinics or in-home iv care), and the risk for rare severe adverse events reported in the literature such as liver damage (47), arrhythmias, and even death (48).
Orbital radiation did not result in better outcome when compared with sham radiation for the primary outcome. The only outcome in which orbital radiotherapy was demonstrated to be superior to sham radiation was the response rate for diplopia. The fact that low-dose radiation was as effective as the standard dose (20 Gy in most studies) may serve as another marker of its limited efficacy in this setting. The combination of radiotherapy with corticosteroids proved to be more effective than either treatment alone. The combination of radiotherapy with corticosteroids was most effective when corticosteroids were given iv, followed by the oral route and retrobulbar administration. However, the combination of iv corticosteroids and radiotherapy was not compared with iv steroids alone, which are currently the treatment of choice according to the EUGOGO recommendations. One previous systematic review published in 2008 by the American Academy of Ophthalmology (49) and one metaanalysis published the same year (50) evaluated the role of orbital radiotherapy in the treatment of GO, with conflicting results. Both included data from observational studies but differed in methodological issues and trials included for analysis. Bradley et al. (49) concluded that orbital radiotherapy had limited role in non-sight-threatening GO, based on highest-quality RCT evidence, whereas Wei et al. (50) found orbital radiotherapy to be effective therapy for GO, based on combined results of RCTs with observational studies. The safety of orbital radiotherapy was demonstrated in studies with up to 29 yr of follow-up (51–53). In these studies no secondary orbital tumors were reported, and low rates of definite retinopathy were documented, mostly in diabetic patients. In the systematic review by Bradley et al. (49), the reported complication rate was 1–2% for radiation retinopathy in the first 10 yr after treatment. When analyzing these studies as well as our report, the efficacy of orbital radiotherapy as monotherapy for patients with GO remains uncertain.
Somatostatin analogs, given as long-acting formulations, showed a statistically significant advantage over placebo in moderately severe GO. However, the effect estimate favoring this treatment was small, reflecting clinical improvement of marginal significance, with common mild gastrointestinal side effects. Three of four trials were sponsored by pharmaceutical companies. Given the high cost of this treatment and in light of its minor clinical efficacy, somatostatin analogs cannot be currently recommended for routine treatment of GO.
Decompression surgery, which constitutes an important treatment mode in severe GO, was compared with iv corticosteroids as first-line therapy for patients with optic neuropathy. In this setting, there was no advantage of surgery over steroid therapy. However, decompression surgery is usually used as a second-line therapy after failure of corticosteroids or need to avoid steroid complications in selected patients, and for this setting no RCT compared surgical with nonsurgical approach.
Additional treatments have been evaluated in patients with GO, with marginal or unproven efficacy. These include IVIG, cyclosporine, colchicine, pentoxifylline, and ongoing trials evaluating rituximab and methotrexate (54, 55). None of these therapies are in routine use for patients with GO, and future studies may help define their role in this condition.
The results presented here provide an evidence base for treatment modalities for GO. However, two other questions often arise regarding eye involvement in Graves’ disease. These include: what factors might exacerbate ophthalmopathy, and how one can prevent its appearance? Evaluation of these questions typically requires study designs other than those included here, including observational studies or analysis of secondary outcomes in RCTs. Examples include observational studies on the effect of smoking on GO (56), and secondary outcome analysis on the effect of iodine-131 (given for hyperthyroidism) on GO course (57). As in many other conditions, regimens used for treatment of GO are different from those used for its prevention and from exacerbating factors. Thus, the effects of smoking, I-131, thyroidectomy, and other factors on the course of GO are beyond the scope of this review.
Our systematic review was limited by several factors. First, most trials evaluated multiple outcome measures, without a clear definition of a primary outcome or used composite end points. This, in combination with small sample sizes, increase the risk for α-type error, finding false statistical differences between treatment groups. Second, several grading systems have been used to evaluate the overall status of GO. These include CAS, OI, TES, NOSPECS, and composite end points (14). Each of these grading systems has limitations in describing GO status (58). To evaluate the efficacy of a specific treatment regimen, a grading system that provides a comprehensive evaluation of the disease status and that enables statistical analysis is needed. The CAS scoring system evaluates disease activity and response to immunosuppressive therapy but does not fully describes the overall status of GO, especially in the seven-item form. However, there is no currently available scoring system that enables both comprehensive evaluation of GO and that results in numerical values allowing statistical analysis. The new vision, inflammation, strabismus, appearance classification system (59) may better reflect the overall status of patients with GO but needs validation before it can be used in clinical trials. Third, there is a known interobserver variability in the evaluation of soft tissue involvement in patients with GO. The use of color slides has been shown (60) to provide a more reliable way to assess treatment effect. This method was reported in only four included trials. Fourth, the majority of studies included in this work evaluated patients with moderately severe active GO without optic neuropathy. The definition of moderately severe disease varied among trials, but most were in accordance with the definition by EUGOGO (6, 7): patients without sight-threatening GO whose eye disease has sufficient impact on daily life to justify the risks of immunosuppression or surgical intervention. There are unanswered questions regarding the optimal management of patients with optic neuropathy as well as lacking data on management of patients with mild active GO. Finally, the natural history of GO is incompletely defined, but in many instances the disease may remit or improve spontaneously, which might affect results in an unpredictable manner. The use of metaanalysis may partially overcome this difficulty by enlarging the sample size.
In conclusion, current evidence demonstrates the efficacy of iv corticosteroids in decreasing CAS in patients with moderate to severe GO. Intravenous pulse corticosteroids have a small but statistically significant advantage compared with oral corticosteroids and causes significantly fewer adverse events. However, this treatment is limited by cost and risk for rare severe adverse events. Somatostatin analogs have marginal clinical efficacy and are expensive. The efficacy of orbital radiotherapy as single therapy remains unclear, whereas the combination of radiotherapy with corticosteroids has better efficacy than either radiotherapy or oral corticosteroids alone.
Acknowledgements
Disclosure Summary: The authors have nothing to disclose.
We thank the following authors for providing additional data for this work: T. C. Chang, M. Stan, J. Gerling, A. Kung, I. Wakelkamp, F. Stamato, and J. Ng.
Abbreviations
- CAS
Clinical activity score
- CI
confidence interval
- EUGOGO
European Group on GO
- GO
Graves’ ophthalmopathy
- IVIG
iv immunoglobulin
- LAR
long-acting release
- NOSPECS
no signs or symptoms, only signs, soft tissue involvement, proptosis, extraocular muscle involvement, corneal involvement and sight loss, graded as O, A, B, or C
- OI
ophthalmopathy index
- OR
odds ratio
- RCT
randomized, controlled trial
- SMD
standardized mean difference
- SR
slow release
- TES
total eye score.
References
van