-
PDF
- Split View
-
Views
-
Cite
Cite
John P. Bilezikian, Maria Luisa Brandi, Richard Eastell, Shonni J. Silverberg, Robert Udelsman, Claudio Marcocci, John T. Potts, Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary Statement from the Fourth International Workshop, The Journal of Clinical Endocrinology & Metabolism, Volume 99, Issue 10, 1 October 2014, Pages 3561–3569, https://doi.org/10.1210/jc.2014-1413
Close - Share Icon Share
Asymptomatic primary hyperparathyroidism (PHPT) is routinely encountered in clinical practices of endocrinology throughout the world. This report distills an update of current information about diagnostics, clinical features, and management of this disease into a set of revised guidelines.
Participants, representing an international constituency, with interest and expertise in various facets of asymptomatic PHPT constituted four Workshop Panels that developed key questions to be addressed. They then convened in an open 3-day conference September 19–21, 2013, in Florence, Italy, when a series of presentations and discussions addressed these questions. A smaller subcommittee, the Expert Panel, then met in closed session to reach an evidence-based consensus on how to address the questions and data that were aired in the open forum.
Preceding the conference, each question was addressed by a relevant, extensive literature search. All presentations and deliberations of the Workshop Panels and the Expert Panel were based upon the latest information gleaned from this literature search.
The expert panel considered all the evidence provided by the individual Workshop Panels and then came to consensus.
In view of new findings since the last International Workshop on the Management of Asymptomatic PHPT, guidelines for management have been revised. The revised guidelines include: 1) recommendations for more extensive evaluation of the skeletal and renal systems; 2) skeletal and/or renal involvement as determined by further evaluation to become part of the guidelines for surgery; and 3) more specific guidelines for monitoring those who do not meet guidelines for parathyroid surgery. These guidelines should help endocrinologists and surgeons caring for patients with PHPT. A blueprint for future research is proposed to foster additional investigation into issues that remain uncertain or controversial.
This Workshop was focused upon patients with primary hyperparathyroidism (PHPT) who lack specific symptoms or signs that are traditionally associated with PTH excess or hypercalcemia. These individuals, who form most PHPT cohorts in most of the world, describe the form known as asymptomatic PHPT. This Workshop did not deal with subjects who have specific signs and symptoms of the disease, in whom surgery is indicated. Since the last International Workshop on this subject in 2008 (1), evolving diagnostic features and new clinical information are changing our views on when parathyroid surgery should be recommended and how to manage those who do not undergo parathyroid surgery. In some patients with asymptomatic disease, surgery is not mandatory. On the other hand, even in these subjects who don't meet any criteria for parathyroidectomy, surgery is always an option because it is the only definitive therapy for PHPT. Guidelines for recommending a surgical approach are provided (Table 1), along with recommendations for monitoring those who do not undergo parathyroid surgery (Table 2).
Guidelines for Surgery in Asymptomatic PHPT: A Comparison of Current Recommendations With Previous Onesa
| . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Measurementb | ||||
| Serum calcium (>upper limit of normal) | 1–1.6 mg/dL (0.25–0.4 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) |
| Skeletal | BMD by DXA: Z-score <−2.0 (site unspecified) | BMD by DXA: T-score <−2.5 at any siteb | BMD by DXA: T-score <−2.5 at any siteb | A. BMD by DXA: T-score < −2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radiusb |
| Previous fragility fracturec | B. Vertebral fracture by x-ray, CT, MRI, or VFA | |||
| Renal | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR < 60 cc/min B. 24-h urine for calcium not recommended | A. Creatinine clearance < 60 cc/min B. 24-h urine for calcium >400 mg/d (>10 mmol/d) and increased stone risk by biochemical stone risk analysisd C. Presence of nephrolithiasis or nephrocalcinosis by x-ray, ultrasound, or CT |
| Age, y | <50 | <50 | <50 | <50 |
| . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Measurementb | ||||
| Serum calcium (>upper limit of normal) | 1–1.6 mg/dL (0.25–0.4 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) |
| Skeletal | BMD by DXA: Z-score <−2.0 (site unspecified) | BMD by DXA: T-score <−2.5 at any siteb | BMD by DXA: T-score <−2.5 at any siteb | A. BMD by DXA: T-score < −2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radiusb |
| Previous fragility fracturec | B. Vertebral fracture by x-ray, CT, MRI, or VFA | |||
| Renal | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR < 60 cc/min B. 24-h urine for calcium not recommended | A. Creatinine clearance < 60 cc/min B. 24-h urine for calcium >400 mg/d (>10 mmol/d) and increased stone risk by biochemical stone risk analysisd C. Presence of nephrolithiasis or nephrocalcinosis by x-ray, ultrasound, or CT |
| Age, y | <50 | <50 | <50 | <50 |
Abbreviations: eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging. Patients need to meet only one of these criteria to be advised to have parathyroid surgery. They do not have to meet more than one.
Surgery is also indicated in patients for whom medical surveillance is neither desired nor possible and in patients opting for surgery, in the absence of meeting any guidelines, as long as there are no medical contraindications.
Consistent with the position established by the ISCD, the use of Z-scores instead of T-scores is recommended in evaluating BMD in premenopausal women and men younger than 50 y (11).
The history of a fragility fracture at any site would define someone as having a complication of PHPT, and thus the individual would be automatically considered to be a surgical candidate.
Most clinicians will first obtain a 24-hour urine for calcium excretion. If marked hypercalciuria is present (>400 mg/d [>10 mmol/d]), further evidence of calcium-containing stone risk should be sought by a urinary biochemical stone risk profile, available through most commercial laboratories. In the presence of abnormal findings indicating increased calcium-containing stone risk and marked hypercalciuria, a guideline for surgery is met.
Guidelines for Surgery in Asymptomatic PHPT: A Comparison of Current Recommendations With Previous Onesa
| . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Measurementb | ||||
| Serum calcium (>upper limit of normal) | 1–1.6 mg/dL (0.25–0.4 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) |
| Skeletal | BMD by DXA: Z-score <−2.0 (site unspecified) | BMD by DXA: T-score <−2.5 at any siteb | BMD by DXA: T-score <−2.5 at any siteb | A. BMD by DXA: T-score < −2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radiusb |
| Previous fragility fracturec | B. Vertebral fracture by x-ray, CT, MRI, or VFA | |||
| Renal | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR < 60 cc/min B. 24-h urine for calcium not recommended | A. Creatinine clearance < 60 cc/min B. 24-h urine for calcium >400 mg/d (>10 mmol/d) and increased stone risk by biochemical stone risk analysisd C. Presence of nephrolithiasis or nephrocalcinosis by x-ray, ultrasound, or CT |
| Age, y | <50 | <50 | <50 | <50 |
| . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Measurementb | ||||
| Serum calcium (>upper limit of normal) | 1–1.6 mg/dL (0.25–0.4 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) | 1.0 mg/dL (0.25 mmol/L) |
| Skeletal | BMD by DXA: Z-score <−2.0 (site unspecified) | BMD by DXA: T-score <−2.5 at any siteb | BMD by DXA: T-score <−2.5 at any siteb | A. BMD by DXA: T-score < −2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radiusb |
| Previous fragility fracturec | B. Vertebral fracture by x-ray, CT, MRI, or VFA | |||
| Renal | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR reduced by >30% from expected B. 24-h urine for calcium >400 mg/d (>10 mmol/d) | A. eGFR < 60 cc/min B. 24-h urine for calcium not recommended | A. Creatinine clearance < 60 cc/min B. 24-h urine for calcium >400 mg/d (>10 mmol/d) and increased stone risk by biochemical stone risk analysisd C. Presence of nephrolithiasis or nephrocalcinosis by x-ray, ultrasound, or CT |
| Age, y | <50 | <50 | <50 | <50 |
Abbreviations: eGFR, estimated glomerular filtration rate; MRI, magnetic resonance imaging. Patients need to meet only one of these criteria to be advised to have parathyroid surgery. They do not have to meet more than one.
Surgery is also indicated in patients for whom medical surveillance is neither desired nor possible and in patients opting for surgery, in the absence of meeting any guidelines, as long as there are no medical contraindications.
Consistent with the position established by the ISCD, the use of Z-scores instead of T-scores is recommended in evaluating BMD in premenopausal women and men younger than 50 y (11).
The history of a fragility fracture at any site would define someone as having a complication of PHPT, and thus the individual would be automatically considered to be a surgical candidate.
Most clinicians will first obtain a 24-hour urine for calcium excretion. If marked hypercalciuria is present (>400 mg/d [>10 mmol/d]), further evidence of calcium-containing stone risk should be sought by a urinary biochemical stone risk profile, available through most commercial laboratories. In the presence of abnormal findings indicating increased calcium-containing stone risk and marked hypercalciuria, a guideline for surgery is met.
Guidelines for Monitoring Patients with Asymptomatic PHPT Who Do Not Undergo Parathyroid Surgery: A Comparison of Current Recommendations With Previous Onesa
| Measurement . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Serum calcium | Biannually | Biannually | Annually | Annually |
| Skeletal | DXA, annually (forearm) | DXA, annually (3 sites) | DXA, every 1–2y (3 sites)a | Every 1–2 y (3 sites),a x-ray or VFA of spine if clinically indicated (eg, height loss, back pain) |
| Renal | eGFR, annually; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, annually; serum creatinine, annually. If renal stones suspected, 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT |
| Measurement . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Serum calcium | Biannually | Biannually | Annually | Annually |
| Skeletal | DXA, annually (forearm) | DXA, annually (3 sites) | DXA, every 1–2y (3 sites)a | Every 1–2 y (3 sites),a x-ray or VFA of spine if clinically indicated (eg, height loss, back pain) |
| Renal | eGFR, annually; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, annually; serum creatinine, annually. If renal stones suspected, 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT |
Abbreviation: eGFR, estimated glomerular filtration rate.
This recommendation acknowledges country-specific advisories as well as the need for more frequent monitoring if the clinical situation is appropriate.
Guidelines for Monitoring Patients with Asymptomatic PHPT Who Do Not Undergo Parathyroid Surgery: A Comparison of Current Recommendations With Previous Onesa
| Measurement . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Serum calcium | Biannually | Biannually | Annually | Annually |
| Skeletal | DXA, annually (forearm) | DXA, annually (3 sites) | DXA, every 1–2y (3 sites)a | Every 1–2 y (3 sites),a x-ray or VFA of spine if clinically indicated (eg, height loss, back pain) |
| Renal | eGFR, annually; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, annually; serum creatinine, annually. If renal stones suspected, 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT |
| Measurement . | 1990 . | 2002 . | 2008 . | 2013 . |
|---|---|---|---|---|
| Serum calcium | Biannually | Biannually | Annually | Annually |
| Skeletal | DXA, annually (forearm) | DXA, annually (3 sites) | DXA, every 1–2y (3 sites)a | Every 1–2 y (3 sites),a x-ray or VFA of spine if clinically indicated (eg, height loss, back pain) |
| Renal | eGFR, annually; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, not recommended; serum creatinine, annually | eGFR, annually; serum creatinine, annually. If renal stones suspected, 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT |
Abbreviation: eGFR, estimated glomerular filtration rate.
This recommendation acknowledges country-specific advisories as well as the need for more frequent monitoring if the clinical situation is appropriate.
Materials and Methods
Experts acknowledged by their major contributions to our recent understanding of asymptomatic PHPT were selected by the organizers of the Workshop (J.P.B., M.L.B., J.T.P.) and invited to participate in the proceedings. Four Workshops were constituted, each headed by one of the invited participants. Based upon the previous Workshop, questions were formulated to be addressed by an extensive review of the peer-reviewed literature. Workshop presentations were based upon these questions and the evidence gleaned from this review that occurred before the Workshop itself. Evidenced-based manuscripts were drafted by each of the Workshop panels and revised in accordance with the results of the Workshop Proceedings, and the consensus achieved by the Expert Panel constitutes the authorship of this report. We endeavored to bear in mind that this report is designed to provide practical guidance for practitioners who care for patients with asymptomatic PHPT.
The evidence upon which the guidelines are based is documented in the four papers that follow this summary statement (2–5). The individual reports address 25 questions that were developed, investigated, and discussed by participants. The four Workshop Panels and the questions they addressed are noted here.
Workshop Group 1: Diagnosis of PHPT (questions 1–3)
Question 1: A, Do we now have optimal reference intervals for serum PTH, and are these intervals based on individuals who are vitamin D replete? B, Do third-generation PTH assays perform better clinically than second-generation PTH assays for the diagnosis of PHPT? C, Is normocalcemic PHPT a part of the diagnostic spectrum of PHPT?
Question 2: A, Should we measure 25-hydroxyvitamin D [25(OH)D] in all patients with suspected PHPT? B, How should the reference ranges for different assays be interpreted? C, What represents the threshold for overtreatment? D, Is it useful to measure 1,25-dihydroxyvitamin D in patients with PHPT, and under what circumstances?
Question 3: A, What is the genetic basis for syndromic and nonsyndromic forms of PHPT? B, What is the value of genetic testing in clinical practice? C, What should be the clinical approach to gene testing in a patient with hypercalcemia?
Workshop Group 2: Presentation of PHPT (questions 4–10)
Question 4: What is known about the natural history of asymptomatic PHPT (both hypercalcemic and normocalcemic), and how do these data help to determine when surgical therapy is appropriate in each variant of the disease?
Question 5: Should regional geographic differences in the clinical presentation of PHPT lead to regional differences in surgical guidelines?
Question 6: Classical features—skeletal. Are there new data on the skeleton in asymptomatic PHPT? A, Do the new data on the skeletal manifestations of PHPT support a re-examination of skeletal criteria for surgery? B, Do the new data warrant a change in guidelines for monitoring with or without parathyroid surgery? C, Should there be a change in the densitometric threshold for surgery? D, Should bone mineral density (BMD) at the lumbar spine, hip, and distal 1/3 radius sites be viewed differently with regard to recommendations for surgery? E, Is there a role for fracture risk assessment tools such as FRAX (6) to assess fracture risk in patients with PHPT, and should this be included in the recommendations for surgery?
Question 7: Classical features—renal. Are there new data on the renal manifestations of PHPT (stones, urinary calcium excretion, renal function)?
Question 8: Nonclassical features—cardiovascular. Are there cardiovascular abnormalities in asymptomatic PHPT?
Question 9: Nonclassical features—neurocognitive. Can neurocognitive dysfunction be detected in asymptomatic PHPT? What is the evidence for a causal relationship?
Question 10: For the nonsurgical management of asymptomatic PHPT, what represents a clinically important change in serum calcium, renal function, and BMD, considering the long-term variability in these parameters?
Workshop Group 3: Surgery for PHPT (questions 11–18)
Question 11: What are the indications for surgery?
Question 12: What is the appropriate preoperative evaluation?
Question 13: What is the appropriate biochemical confirmation?
Question 14: What are the appropriate preoperative imaging studies?
Question 15: What are the operative options?
Question 16: How and when should intraoperative PTH assays be employed?
Question 17: Are there special considerations for surgery for hereditary syndromes?
Question 18: What are the complications of parathyroid surgery?
Workshop Group 4: Medical management of PHPT (questions 19–25)
Question 19: Should the recommendation for calcium intake in patients with PHPT differ from that of the general population?
Question 20: Do patients with PHPT who are insufficient or deficient in vitamin D benefit from supplementation? How should vitamin D deficiency/insufficiency be managed in patients with PHPT?
Question 21: How effective is estrogen receptor-targeted therapy in postmenopausal women with PHPT?
Question 22: What is the efficacy and safety of bisphosphonate therapy in men and women with PHPT?
Question 23: Is therapy with cinacalcet effective and safe in patients with PHPT across the spectrum of serum calcium concentrations? Is it cost-effective?
Question 24: Is therapy with cinacalcet effective in patients with familial PHPT?
Question 25: Can cinacalcet and bisphosphonate be combined in patients with PHPT?
These questions are addressed in the individual source documents that accompany this statement (2–5).
This Guidelines Statement summarizes the conclusions of the Workshop Panels as adopted by the Expert Panel. The document presents a spectrum of evidence that has given new insights into certain features of the disease, but admittedly falls short of complete knowledge. Areas for which more data are needed are indicated in this paper as well as in the individual articles.
Diagnosis of Primary Hyperparathyroidism
Hypercalcemia is documented in patients with classical PHPT. The total serum calcium concentration is adjusted to reflect any abnormality in albumin, the major calcium binding protein. The formula to use is: corrected calcium = measured total serum calcium in mg/dL + 0.8 × (4.0 − patient's serum albumin concentration in g/dL). Ionized serum calcium can be measured, but most centers do not have sufficient capabilities to rely upon an ionized, free calcium concentration. Thus, the corrected total serum calcium concentration is recommended.
Second- and third-generation assays for PTH are equivalently useful in the diagnosis of PHPT. The normal reference range is influenced by the extent of vitamin D repletion, although the extent of vitamin D repletion is also controversial in view of the recommended threshold value of the Institute of Medicine (IOM) for 25(OH)D of 20 ng/mL (50 nmol/L) and other authoritative groups like The Endocrine Society recommending a threshold value of 30 ng/mL (75 nmol/L) (7, 8). Studies in populations that are vitamin D replete, in general, give normal ranges that are lower than reference ranges that are not defined by vitamin D adequacy. The percentage of subjects with PTH levels, by either generation assay, that are technically in the normal reference range—although abnormal for the presence of hypercalcemia—may be a function of the vitamin D adequacy of the cohort. In subjects whose 25(OH)D levels are above 30 ng/mL, a greater percentage will have levels of PTH within the posted laboratory reference range. A reference range for PTH in subjects with normal vitamin D levels has not yet been established, but it is an area for future research.
Normocalcemic PHPT is now a well-recognized variant of PHPT. These subjects have normal total and ionized serum calcium levels without any known etiologies for a secondary elevation of PTH. Knowledge of the natural history of normocalcemic PHPT is incomplete, but some individuals become hypercalcemic, and some show evidence of target organ involvement (eg, reduced BMD). Others, however, appear to be stable over time with persistently elevated PTH levels and normal serum calcium concentrations.
25(OH)D should be measured in all subjects with PHPT. There is evidence that the disease is more active when subjects are vitamin D insufficient (<20 ng/mL) or frankly deficient (<10 ng/mL), as defined by the IOM (9). There is also evidence that reductions in PTH levels can occur when insufficient levels are corrected, with lower PTH levels reported when insufficient levels of 25(OH)D in PHPT are corrected (9). Although 1,25-dihydroxyvitamin D levels are typically at the upper limits of normal, and occasionally elevated, there does not seem to be any value in measuring this active metabolite. It is therefore not recommended.
Advances in genetics have led to an estimate that >10% of patients with PHPT harbor a mutation in one of 11 genes that have been associated with syndromic and nonsyndromic forms of PHPT. As the cost of genetic testing continues to decline, such testing is likely to be utilized more routinely, thus helping in the clinical management and treatment of patients with PHPT and their relatives (see Refs. 2 and 4 for more extensive discussions).
Classical Presentations of Primary Hyperparathyroidism
The natural history of PHPT suggests that whereas asymptomatic subjects are usually stable, they may not be stable indefinitely. Progression can occur, such that by 15 years of prospective follow-up, as many as one-third of subjects will demonstrate more overt features of the disease (eg, kidney stones, worsening hypercalcemia, reduced BMD) (10). Although the disease presents with some variability in terms of symptomatology (more symptomatic in Asia and Latin America than in Europe or in North America), these observations do not suggest a region-specific approach to the disease when it presents asymptomatically.
The skeleton
Newer approaches to skeletal evaluation suggest that dual-energy x-ray absorptiometry (DXA), although extremely useful, may not give complete information about the trabecular compartment of bone in PHPT. As a marker of cortical disease, however, DXA is informative, emphasizing the importance of measuring the distal 1/3 radius, a cortical site, in all patients with PHPT. Other modalities, such as vertebral fracture assessment (VFA) and trabecular bone score (TBS) by DXA, and high-resolution peripheral quantitative computed tomography (HRpQCT) all can help to substantiate trabecular involvement in many patients with asymptomatic PHPT. More global skeletal involvement, as suggested by application of these newer imaging modalities, is supported by generally increased bone turnover markers in PHPT. Data on fracture incidence in PHPT, albeit in most cases retrospective, support the concept that in PHPT, fracture risk is increased at both nonvertebral (cortical) and vertebral (trabecular) sites. An area for future research is to determine whether a fracture risk assessment tool, such as FRAX, can be used to predict fracture risk in PHPT.
The kidney
The kidney is a principal target of PHPT. Renal stone disease and nephrocalcinosis are the most common overt complications of PHPT. Stone disease is multifactorial and is not clearly accounted for on the basis of urinary calcium excretion alone. On the other hand, a complete urinary evaluation for stone risk as well as a renal imaging modality (abdominal x-ray, ultrasound, or computed tomography [CT]) to determine whether or not nephrocalcinosis or asymptomatic nephrolithiasis is present could identify a cohort at higher risk. This more extensive renal evaluation is now recommended (Table 1). Patients who demonstrate marked hypercalciuria on a normal calcium diet should be further evaluated for calcium-stone risk by a more complete stone risk profile. A 24-hour urine analysis for ingredients of calcium-containing stones is available from most commercial laboratories. Patients who demonstrate marked hypocalciuria on a normal calcium diet might have a form of familial hypocalciuric hypercalcemia (FHH; see Ref. 2). Patients who demonstrate a urinary calcium:creatinine clearance ratio <0.01 should also be evaluated further for the possibility of FHH (2).
Nonclassical Manifestations Sometimes Attributed to PHPT
Cardiovascular
Although there is evidence, often subtle, for vascular and cardiovascular dysfunction in some patients with mild PHPT, it is unclear whether these observations have predictive value. At this time, there are no prospective data on cardiovascular outcomes in mild PHPT. Moreover, studies that have tracked cardiovascular endpoints before and after surgery have not shown any benefit when the studies have been rigorously conducted in randomized controlled trials. A reasonable conclusion to be drawn at this time, therefore, is that tests for cardiovascular involvement should not be part of the evaluation in PHPT, and that parathyroid surgery should not be performed to improve cardiovascular endpoints, if abnormalities are present.
Neurocognitive
Symptoms volunteered by patients or elicited by health care professionals have called attention to nonspecific symptomatology such as the lack of mental focus, changes in cognition, depression, and overall reduced quality of life. Data from recently reported studies designed to determine whether these features are directly related to PHPT are not consistent. After parathyroidectomy, inconsistent results are also seen. Until more definitive evidence becomes available, such nonspecific symptomatology should not be used to recommend parathyroidectomy. Nonetheless, some patients with neurocognitive symptoms do appear to benefit from surgical intervention. Formal neuropsychiatric or neurocognitive testing in PHPT, although appropriate for a research agenda in this disease, is not recommended.
Management
A recommended approach to evaluating the patient with asymptomatic PHPT is listed in Table 3. For patients who do not undergo parathyroid surgery, monitoring is indicated (Table 2).
Recommendations for the Evaluation of Patients With Asymptomatic PHPT
| Recommended |
| Biochemistry panel (calcium, phosphate, alkaline phosphatase activity, BUN, creatinine), 25(OH)D |
| PTH by second- or third-generation immunoassay |
| BMD by DXA |
| Lumbar spine, hip, and distal 1/3 radius |
| Vertebral spine assessment |
| X-ray or VFA by DXA |
| 24-h urine for: |
| Calcium, creatinine, creatinine clearance |
| Stone risk profile |
| Abdominal imaging by x-ray, ultrasound, or CT scan |
| Optional |
| HRpQCT |
| TBS by DXA |
| Bone turnover markers (bone-specific alkaline phosphatase activity, osteocalcin, P1NP [select one]; serum CTX, urinary NTX [select one]) |
| Fractional excretion of calcium on timed urine sample |
| DNA testing if genetic basis for PHPT is suspected |
| Recommended |
| Biochemistry panel (calcium, phosphate, alkaline phosphatase activity, BUN, creatinine), 25(OH)D |
| PTH by second- or third-generation immunoassay |
| BMD by DXA |
| Lumbar spine, hip, and distal 1/3 radius |
| Vertebral spine assessment |
| X-ray or VFA by DXA |
| 24-h urine for: |
| Calcium, creatinine, creatinine clearance |
| Stone risk profile |
| Abdominal imaging by x-ray, ultrasound, or CT scan |
| Optional |
| HRpQCT |
| TBS by DXA |
| Bone turnover markers (bone-specific alkaline phosphatase activity, osteocalcin, P1NP [select one]; serum CTX, urinary NTX [select one]) |
| Fractional excretion of calcium on timed urine sample |
| DNA testing if genetic basis for PHPT is suspected |
Abbreviations: BUN, blood urea nitrogen; P1NP, procollagen type 1 N-propeptide; CTX, C-telopeptide cross-linked collagen type I; NTX, N-telopeptide of type I collagen. This evaluation is for PHPT, not to distinguish between PHPT and other causes of hypercalcemia.
Recommendations for the Evaluation of Patients With Asymptomatic PHPT
| Recommended |
| Biochemistry panel (calcium, phosphate, alkaline phosphatase activity, BUN, creatinine), 25(OH)D |
| PTH by second- or third-generation immunoassay |
| BMD by DXA |
| Lumbar spine, hip, and distal 1/3 radius |
| Vertebral spine assessment |
| X-ray or VFA by DXA |
| 24-h urine for: |
| Calcium, creatinine, creatinine clearance |
| Stone risk profile |
| Abdominal imaging by x-ray, ultrasound, or CT scan |
| Optional |
| HRpQCT |
| TBS by DXA |
| Bone turnover markers (bone-specific alkaline phosphatase activity, osteocalcin, P1NP [select one]; serum CTX, urinary NTX [select one]) |
| Fractional excretion of calcium on timed urine sample |
| DNA testing if genetic basis for PHPT is suspected |
| Recommended |
| Biochemistry panel (calcium, phosphate, alkaline phosphatase activity, BUN, creatinine), 25(OH)D |
| PTH by second- or third-generation immunoassay |
| BMD by DXA |
| Lumbar spine, hip, and distal 1/3 radius |
| Vertebral spine assessment |
| X-ray or VFA by DXA |
| 24-h urine for: |
| Calcium, creatinine, creatinine clearance |
| Stone risk profile |
| Abdominal imaging by x-ray, ultrasound, or CT scan |
| Optional |
| HRpQCT |
| TBS by DXA |
| Bone turnover markers (bone-specific alkaline phosphatase activity, osteocalcin, P1NP [select one]; serum CTX, urinary NTX [select one]) |
| Fractional excretion of calcium on timed urine sample |
| DNA testing if genetic basis for PHPT is suspected |
Abbreviations: BUN, blood urea nitrogen; P1NP, procollagen type 1 N-propeptide; CTX, C-telopeptide cross-linked collagen type I; NTX, N-telopeptide of type I collagen. This evaluation is for PHPT, not to distinguish between PHPT and other causes of hypercalcemia.
Monitoring is focused on detecting changes in the serum calcium concentration, significant reductions in BMD, occurrence of a fragility fracture, or changes in renal endpoints.
Guidelines for surgery
The threshold value for the serum calcium above which surgery is recommended remains >1 mg/dL (>0.25 mm/L) above the upper limit of normal.
Skeletal guidelines
- •
BMD: Reductions in bone density continue to be a cause for concern in PHPT, either as patients present or as they are monitored. Surgery is recommended for peri- or postmenopausal women and men age 50 and older who have a T-score of −2.5 or less at the lumbar spine, femoral neck, total hip, or distal 1/3 radius. In premenopausal women and in men under 50, the Z-score of ≤−2.5 is recommended as the cut-point below which surgery is advised. The use of Z-scores instead of T-scores is consistent with the International Society of Clinical Densitometry (ISCD) official position in evaluating BMD in this population (11). This recommendation recognizes, however, that in PHPT, other effects of PTH on bone size and structure could influence fracture proclivity. Other approaches to skeletal evaluation, such as vertebral x-ray, VFA, TBS, or HRpQCT may provide information to help in the decision to recommend surgery. Substantial trabecular disease by TBS or HRpQCT could support a recommendation for surgery, recognizing that these modalities are not routinely available and thus cannot be widely used.
- •
Fracture: If a vertebral fracture is present by x-ray or VFA, surgery is recommended, even if there is no prior documentation.
Renal guidelines
- •
Renal function: a creatinine clearance of <60 cc/min continues to constitute a criterion for surgery.
- •
Renal stone evaluation is now recommended by renal imaging with x-ray, ultrasound, or CT. If stones or nephrocalcinosis is present, surgery is recommended.
- •
Twenty-four-hour urine for calcium will help in the differential diagnosis of FHH. If marked hypercalciuria is present (ie, >400 mg/d), a more complete urinary biochemical stone profile should be considered. In the presence of abnormal findings indicating increased calcium-containing stone risk and marked hypercalciuria, a guideline for surgery is met.
Age under 50 continues to be an evidence-based guideline for surgery.
Changes in specific endpoints during monitoring that should lead to a recommendation for parathyroid surgery (Table 4)
Indications for Parathyroid Surgery During Monitoring
| Measurement . | 2013 . |
|---|---|
| Serum calcium (>upper limit of normal) | >1 mg/dL (>0.25 mmol/L) |
| Skeletal | A. T-score <−2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radius; or a significant reduction in BMDa |
| B. Vertebral fracture by x-ray, CT, MRI, or VFA | |
| Renal | A. CrCl < 60 cc/min |
| B. Clinical development of a kidney stone or by imaging (x-ray, ultrasound, or CT) |
| Measurement . | 2013 . |
|---|---|
| Serum calcium (>upper limit of normal) | >1 mg/dL (>0.25 mmol/L) |
| Skeletal | A. T-score <−2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radius; or a significant reduction in BMDa |
| B. Vertebral fracture by x-ray, CT, MRI, or VFA | |
| Renal | A. CrCl < 60 cc/min |
| B. Clinical development of a kidney stone or by imaging (x-ray, ultrasound, or CT) |
Abbreviations: MRI, magnetic resonance imaging; CrCl, creatinine clearance.
A significant change is defined by a reduction that is greater than the least significant change as defined by the International Society for Clinical Densitometry (11).
Indications for Parathyroid Surgery During Monitoring
| Measurement . | 2013 . |
|---|---|
| Serum calcium (>upper limit of normal) | >1 mg/dL (>0.25 mmol/L) |
| Skeletal | A. T-score <−2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radius; or a significant reduction in BMDa |
| B. Vertebral fracture by x-ray, CT, MRI, or VFA | |
| Renal | A. CrCl < 60 cc/min |
| B. Clinical development of a kidney stone or by imaging (x-ray, ultrasound, or CT) |
| Measurement . | 2013 . |
|---|---|
| Serum calcium (>upper limit of normal) | >1 mg/dL (>0.25 mmol/L) |
| Skeletal | A. T-score <−2.5 at lumbar spine, total hip, femoral neck, or distal 1/3 radius; or a significant reduction in BMDa |
| B. Vertebral fracture by x-ray, CT, MRI, or VFA | |
| Renal | A. CrCl < 60 cc/min |
| B. Clinical development of a kidney stone or by imaging (x-ray, ultrasound, or CT) |
Abbreviations: MRI, magnetic resonance imaging; CrCl, creatinine clearance.
A significant change is defined by a reduction that is greater than the least significant change as defined by the International Society for Clinical Densitometry (11).
- •
An increase in serum calcium >1 mg/dL (0.25 mmol/L) above the upper limit of normal.
- •
A reduction in BMD that is significantly decreased over the baseline measurement and a T-score that falls below −2.5 at that site. If the patient demonstrates a progressive reduction in BMD that exceeds the least significant change at any site and the T-score falls to between −2.0 and −2.5, the physician may opt to recommend surgery, although guidelines have not been strictly met.
- •
The occurrence of a fragility fracture.
- •
The occurrence of a kidney stone.
- •
A reduction in creatinine clearance to <60 mL/min.
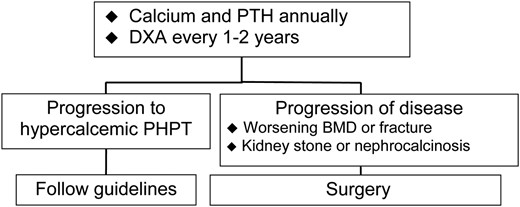
In patients with normocalcemic PHPT, the diagram in Figure 1 presents a reasonable approach to this variant.
Current issues in surgical management of PHPT
The information at the conference, which is summarized above and in the source document (4), points to greater utilization of parathyroidectomy in PHPT now than before. After successful parathyroid surgery, bone density improves, fracture incidence is reduced (cohort studies), kidney stones are reduced in frequency among those with a history of renal stones, and, although not confirmed by randomized clinical trials, there may well be improvements in some neurocognitive elements. With advances in the effectiveness and safety of surgical techniques, particularly in the hands of expert parathyroid surgeons, the decision to remove the abnormal parathyroid tissue is bolstered by added confidence of its success. Furthermore, improvements in a variety of preoperative imaging modalities have given the parathyroid surgeon a road map that is in general very reliable. Imaging is used as an aide to surgery, but not for diagnostic purposes. The most commonly used approaches are radionuclide imaging with 99mTc-sestamibi scan accompanied by single photon emission CT. Ultrasound is also commonly employed as an imaging approach. CT scanning has become more widely used with added sensitivity by employing three-dimensional technology and a fourth dimension (time).
Among a wide variety of surgical approaches, the minimally invasive parathyroidectomy has gained popularity. With intraoperative measurements of PTH, the minimally invasive parathyroidectomy procedure limits the scope and duration of the surgery. The PTH level is expected to fall by 50%, into the normal range, within 10–15 minutes after removal of all hyperfunctioning parathyroid tissue (eg, single adenoma, hyperplastic glands). In the case of genetic forms of PHPT, the surgical approach is tailored to the underlying genetic disorder. Parathyroid surgery should be performed only by surgeons who are highly experienced in this operation.
Current issues in medical management of PHPT
Although the revised guidelines are likely to lead to a recommendation for parathyroid surgery more frequently than the last set of guidelines, asymptomatic patients who do not meet surgical guidelines can be followed safely without surgery, at least for a period of years. There are also individuals who will either refuse surgery or who are not candidates for surgery because of extenuating medical issues. For these individuals, a nonsurgical approach is needed. These patients are monitored (Table 2) with serum calcium measured annually or biannually. BMD is also measured on a regular basis, but the frequency is often based on country-specific guidelines for DXA. The appropriate monitoring interval suitable for individual patients with PHPT is also guided by clinical judgment. In some cases, it might be appropriate to monitor BMD on an annual basis, whereas in others a 2-year schedule might be acceptable. VFA is a recommended approach for monitoring the occurrence of a subclinical vertebral fracture.
All patients who are to be followed without surgery should be replete in vitamin D. Vitamin D should be given in modest amounts to reach a minimum serum level of 25(OH)D >20 ng/dL (50 nmol/L). Generally, 800 to 1000 IU is a useful starting dose. Because there is some evidence that levels >30 ng/mL may be associated with further reductions in PTH levels, one could make a reasonable argument to aim for this higher threshold value. Calcium intake should follow guidelines established for all individuals. It is not recommended to limit calcium intake in PHPT.
Pharmacological approaches are available, although most have not been approved by the Food and Drug Administration or other regulatory agencies. Moreover, for most drugs, long-term data are insufficient regarding benefit and safety. Among the bisphosphonates, the best evidence is for the use of alendronate, which improves BMD at the lumbar spine without altering the serum calcium and PTH concentrations. The calcimimetic cinacalcet reduces the serum calcium concentration to normal in many individuals but has only a modest effect to reduce the PTH level. BMD does not change. Cinacalcet is an approved drug for the medical management of PHPT. There are limited data on the combination of bisphosphonate and cinacalcet in PHPT. The use of any of these pharmacological agents should be dictated by the goal. To increase BMD, bisphosphonate therapy would be the choice. If there is concern about the level of the serum calcium concentration, cinacalcet would be the choice to reduce it. If there is no intention to improve the BMD or to lower the serum calcium concentration, pharmacological agents are not used.
Blueprint for Future Research
The Workshop identified a number of areas that are recommended for more research over the next 5 years. They are listed here as broad categories for further investigation.
- 1.
Normocalcemic PHPT: natural history and pathophysiology.
- 2.
Threshold values for 25(OH)D in defining the normal range of PTH; in defining optimal levels to control PTH levels.
- 3.
Therapeutic regimen(s) to safely replete vitamin D in deficient patients; establishment of a goal to reach in ng/mL (nmol/L) of serum 25(OH)D.
- 4.
Prospective, randomized, controlled cohort studies of nontraditional aspects of PHPT: neurocognitive and vascular function before and after parathyroidectomy. Determination of predictive indices.
- 5.
Fracture incidence in PHPT, before and after successful surgery.
- 6.
Comparison and predictive value of BMD, high-resolution imaging, and their associated analytical analyses (eg, TBS, finite element analysis) to fracture risk in PHPT.
- 7.
Establishment of a FRAX-equivalent tool to assess fracture risk in PHPT.
- 8.
Determination of the incidence of subclinical renal stone disease in asymptomatic PHPT by abdominal imaging.
- 9.
Determination of the predictive value of stone risk in PHPT, as determined by urinary biochemical analyses and the presence and/or development of kidney stones.
- 10.
Pharmacological approaches to PHPT, including combination bisphosphonate and cinacalcet; denosumab: cost-benefit analyses.
Conclusion
The Fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism reviewed evidence that has become available since the last Workshop in 2008. Revised guidelines have been proposed to help the endocrinologist and surgeon decide on the advisability of parathyroid surgery. Meeting a guideline for surgery defines a certain element of increased risk for classical complications of PHPT. Patients who do not meet any guidelines for surgery may appropriately undergo parathyroid surgery as long as there are no medical contraindications. Although surgery is clearly an attractive choice in many subjects with asymptomatic PHPT, those who do not meet surgical indications or are unable or unwilling to proceed with parathyroidectomy can be monitored. If the patient meets surgical guidelines but is not a candidate for parathyroid surgery, specific pharmacological approaches that target either hypercalcemia or reduced bone mass may be of value. The benefits of pharmacological intervention, however, need further evaluation.
Acknowledgments
On behalf of all participants of the fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism: Goran Åkerstrom, Francisco Bandeira, Rocco Ballantone, Carlo Biagini, Jens Bollerslev, Stephanie Boutroy, Bart Clarke, Aline G. Costa, Natalie E. Cusano, Pierre D'Amour, David W. Dempster, Quan-Yang Duh, Guido Gasparri, Aliya Khan, Michael Kleerekoper, Giancarlo Isaia, E. Michael Lewiecki, Jian-min Liu, Paolo Miccoli, Salvatore Minisola, Bruno Niederle, Ranuccio Nuti, Munro Peacock, Lars Rejnmark, Rene Rizzoli, Dolores M. Shoback, Barbara C. Silva, Rajesh V. Thakker, Francesco Tonelli, and Marcella D. Walker.
Disclosure Summary: R.E. provides consulting advice to Roche Diagnostics and Immunodiagnostics Systems. All other authors have nothing to disclose.
Abbreviations
- BMD
bone mineral density
- CT
computed tomography
- DXA
dual-energy x-ray absorptiometry
- FHH
familial hypocalciuric hypercalcemia
- HRpQCT
high-resolution peripheral quantitative computed tomography
- 25(OH)D
25-hydroxyvitamin D
- PHPT
primary hyperparathyroidism
- TBS
trabecular bone score
- VFA
vertebral fracture assessment.
References