Abstract
Crataegus spp. (hawthorn) monopreparations are predominantly used for treating congestive heart failure. The effectiveness of hawthorn preparations (flowers with leaves; berries) is documented in a number of clinical studies, reviews and meta-analyses. The aim of this article is to assess the safety data of all available human studies on hawthorn monopreparations.
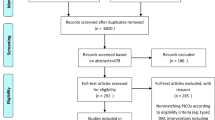
Systematic searches were conducted on MEDLINE, EMBASE, AMED, The Cochrane Library, the UK National Research Register and the US ClinicalTrials.gov (up to January 2005). Data were requested from the spontaneous reporting scheme of the WHO. Hand searches were also conducted in a sample of relevant medical journals, conference proceedings, reference lists of identified articles and our own files. Eight manufacturers of hawthorn-containing preparations were contacted and asked to supply any information on adverse events or drug interactions. Data from all clinical studies and reports were assessed. Only human studies on monopreparations were included. Data from hawthorn-containing combination preparations and homeopathic preparations were excluded. All studies were read and evaluated by one reviewer and independently verified by at least one additional reviewer.
Twenty-nine clinical studies were identified, of which 24 met our inclusion criteria. A total of 7311 patients were enrolled, and data from 5577 patients were available for analysis. The daily dose and duration of treatment with hawthorn monopreparations ranged from 160 to 1800mg and from 3 to 24 weeks, respectively. The extracts most used in the clinical trials were WS 1442 (extract of hawthorn standardised to 18.75% oligomeric procyanidins) and LI 132 (extract of hawthorn standardised to 2.25% flavonoids). Overall, 166 adverse events were reported. Most of these adverse events were, in general, mild to moderate; eight severe adverse events have been reported with the LI 132 extract. The most frequent adverse events were dizziness/vertigo (n = 15), gastrointestinal complaints (n = 24), headache (n = 9), migraine (n = 8) and palpitation (n = 11). The WHO spontaneous reporting scheme received 18 case reports. In the identified trials, the most frequent adverse events were dizziness (n = 6), nausea (n = 5), fall (n = 2), gastrointestinal haemorrhage (n = 2), circulation failure (n = 2) and erythematous rash (n = 2). There were no reports of drug interactions.
In conclusion, all data reviewed in this article seem to indicate that hawthorn is well tolerated even if some severe adverse events were reported; this suggests that further studies are needed to better assess the safety of hawthorn-containing preparations. Moreover, the unsupervised use of this drug can be associated with problems, especially if given with concomitant medications.






Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Chang Q, Zuo Z, Harrison F, et al. Hawthorn. J Clin Pharmacol 2002; 42: 605–12605-12
Cupp MJ. Hawthorn. In: Cupp MJ, Annonn J. Toxicology and clinical pharmacology of herbal products. Totowa (NJ): Humana Press, 2000: 253–8
Williamson EM. British herbal pharmacopoeia, 1983. Bournemouth: British Herbal Medicine Association (BHMA) Publications, 2003
Weiss RF, Fintelmann V. Herbal medicine. New York: Thieme Stuttgart, 2000
Newall A, Anderson LA, Phillipson JD. Herbal medicines: a guide for health-care professionals. London: The Pharmaceutical Press, 1996
Blumenthal M. The ABC clinical guide to herbs. Austin (TX): American Botanical Council, 2003
Blumenthal M, Busse WR, Goldberg A. The complete Commission E monographs. Austin (TX): American Botanical Council, 2000
Schussler M, Holz J, Fricke U. Myocardial effects of flavonoids from Crataegus species. Arzneimittel Forschung 1995; 45: 842–5
Weiss RF, Fintelmann V. Herbal medicine. New York: Thieme Stuttgart, 2000
Rigelsky JM, Sweet BV. Hawthorn: pharmacology and therapeutic uses. Am J Health Syst Pharm 2002; 59: 417–22
Societàl Italiana di Fitoterapia, Organizzazione Mondiale della Sanità (OMS). Monografie di piante medicinali. Vol. 2. Abbiategrasso (Milan), Italy: Le Nuove Scritture, 2004
Pittler MH, Schmidt K, Ernst E. Hawthorn extract for treating chronic heart failure: meta-analysis of randomized trials. Am J Med 2003; 114: 665–74
Reynolds JEJ, editor. Hawthorne. Martindale: the extra pharmacopoeia. London: The Royal Pharmaceutical Society of Great Britain, Pharmaceutical Press, 1996: 1600
Iwamoto M, Ishizaki T, Sato T. Klinische Wirkung von Crataegutt® bei Herzerkrankungen ischa mischer und/oder hypertensiver Genese: Eine multizentrische Doppelblindstudie. Planta Med 1981; 42: 1–16
O’Connolly M, Bernhoft G, Bartsch G. Behandlung alterer, multimorbider Patienten mit stenokardischen Beschwerden: Eine placebokontrollierte crossover-Doppelblindstudie mit Crataegutt® novo. Therapiewoche 1987; 37: 3587–600
O’Connolly M, Jansen W, Bernhôft G, et al. Treatment of decreasing cardiac performance (NYHA stages I to II) in advanced age with standardized crataegus extract. Fortschr Med 1986; 42: 805–8
Hanak T, Brûckel MH. Behandlung von leichten stabilen Formen der Angina pectoris mit Crataegutt novo. Therapiewoche 1983; 33: 4331–3
Weikl A, Assmus KD, Neukum-Schmidt A. Objective confirmation of the efficacy of a special crataegus extract WS1442 in patients with cardiac insufficiency (NYHA II). Fortschr Med 1996; 114: 291–6
Eichstâdt H, Stôrk T, Môckel M. Wirksamkeit und Vertrâglichkeit von Crataegus-Extrakt WS®1442 bei herzinsuffizienten Patienten mit eingeschrankter linksventrikulârer Funktion. Perfusion 2001; 14: 212–7
Leuchtgens H. The crataegus special extract WS 1442 in patients with cardiac insufficiency NYHA II: a placebo-controlled double-blind study. Fortschr Med 1993; 111: 352–4
Zapfe G. Clinical efficacy of crataegus extract WS®1442 in congestive heart failure NYHA class II. Phytomedicine 2001; 8: 262–6
Tauchert M. Efficacy and safety of crataegus extract WS®1442 in comparison with placebo in patients with chronic stable New York Heart Association class-III heart failure. Am Heart J 2002; 143: 910–5
Staiger J, Kuhn H, Spâth J. Zur kardialen Wirksamkeit von low-dose digitoxin (0.07 mg) and crataegus. Med Welt 1987; 38: 1023–8
Eichstâdt H, Bâder M, Danne O. Crataegus-Extrakt hilft dem Patienten mit NYHA II-Herzinsuffizienz. Therapiewoche 1989; 39: 3288–96
Weikl A, Noh HS. Der Einfluss von Crataegus bei globaler Herzinsuffizienz. Herz Gefâsse 1992; 12: 6–24
Tauchert M, Gildor A, Lipinski J. High-dose crataegus (hawthorn) extract WS®1442 in the treatment of NYHA stage II heart failure. Herz 1999; 24: 465–74
Habs M. Prospective, comparative cohort studies and their contribution to the benefit assessment of therapeutic options: heart failure treatment with and without hawthorn special extract WS 1442. Forsch Komplementârmed Klass Naturheilkd 2004; 11: S36–9
Alexander A. Klinische Wirkung des Crataegus Extraktes LI132 bei der Therapie der Herzinsuffizienz im Stadium II der New York Heart Association. Eine randomisierte, plazebokontrollierte Doppelblindstudie an N = 73 Patienten [doctoral thesis; online]. Available from URL: http://edoc.hu-berlin.de/docviews/abstract.php?lang=ger&id=10095 [Accessed 2006 Jan 27]
Bôadigheimer K, Chasa D. Effectiveness of hawthorn extract at a dosage of 3 × 100mg per day: multicentre double-blind trial with 85 NYHA stage II heart failure patients. Mûanch Med Wochenschr 1994; 136: S7–S11
Schmidt U, Kuhn U, Ploch M, et al. Efficacy of hawthorn (crataegus) preparation LI132 in 78 patients with chronic congestive heart failure defined as NYHA functional class II. Phytomedicine 1994; 1: 17–24
Tauchert M, Ploch M, Hubner WD. Effectiveness of the hawthorn extract LI 132 compared with the ACE inhibitor captopril. Mûanch Med Wochenschr 1994; 136: S27–33
Fischer K, Jung F, Koscielny J, et al. Crataegus-extract vs methyldidoxin. Mûanch Med Wochenschr 1994; 136: S35–8
Fôrster A, Fôrster K, Bûhring M, et al. Crataegus bei mâβiger reduzierter linksventrikulârer Auswurffraktion. Mûanch Med Wochenschr 1994; 136: S21–6
Schmidt U, Albrecht M, Podzuweit H. High dosed therapy with crataegus extract in patients suffering from heart failure NYHA stage I and II. Z Phytother 1998; 19: 22–30
Rietbrock N, Hamel M, Hempel B. Efficacy of a standardized extract of fresh crataegus berries on exercise tolerance and quality of life in patients with congestive heart failure (NYHA II). Arzneimittel Forschung 2001; 51: 793–8
Degenring FH, Suter A, Weber M, et al. A randomised double blind placebo controlled clinical trial of a standardised extract of fresh crataegus berries (Crataegisan®) in the treatment of patients with congestive heart failure NYHA II. Phytomedicine 2003; 10: 363–9
Walker AF, Marakis G, Morris AP, et al. Promising hypotensive effect of hawthorn extract: a randomised double-blind pilot study of mild, essential hypertension. Phytother Res 2002; 16: 48–54
Barnes J, Mills SY, Abbot NC. Different standards of reporting of ADRs to herbal remedies and conventional OTC medicines: face to face interviews with 515 users of herbal remedies. Br J Clin Pharmacol 1998; 45: 496–500
Ernst E, Pittler MH, Stevinson C, et al. The desktop guide to complementary and alternative medicine: an evidence based approach. Edinburgh: Mosby, 2001
Tankanow R, Tamer HR, Streetman DS, et al. Interaction study between digoxin and a preparation of hawthorn (Crataegus oxyacantha). Clin Pharmacol 2003; 43: 637–42
Mills S, Bone K. The essential guide to herbal safety. Edinburgh: Elsevier Churchill Livingstone, 2005
Acknowledgements
The authors wish to thank Dr Antonella Di Sotto, Department of Pharmacology of Natural Substance and General Physiology, University of Rome, ‘La Sapienza’, Rome, Italy, for her support.
No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Daniele, C., Mazzanti, G., Pittler, M.H. et al. Adverse-Event Profile of Crataegus Spp.. Drug-Safety 29, 523–535 (2006). https://doi.org/10.2165/00002018-200629060-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200629060-00005