Abstract
Drug-induced liver injury is a frequent cause of hepatic dysfunction. Reliably establishing whether the liver disease was caused by a drug requires the exclusion of other plausible causes and the search for a clinical drug signature. The drug signature consists of the pattern of liver test abnormality, the duration of latency to symptomatic presentation, the presence or absence of immune-mediated hypersensitivity and the response to drug withdrawal.
Determination of causality also includes an evaluation of individual susceptibility to drug-induced liver injury. This susceptibility is governed by both genetic and environmental factors. Components of the drug signature in conjunction with certain risk factors have been incorporated into formal scoring systems that are predictive of the likelihood of drug-induced liver injury. The most validated scoring system is the Roussel-Uclaf causality assessment method, which nonetheless retains certain imperfections.
Mitigating the potential for drug-induced liver injury is achieved by the identification of toxicity signals during clinical trials and the monitoring of liver tests in clinical practice. There are three signals of liver toxicity in clinical trials: (i) a statistically significant doubling (or more) in the incidence of serum alanine aminotransferase (ALT) elevation >3 × the upper limit of normal (ULN); (ii) any incidence of serum ALT elevation >8–10 × ULN; and (iii) any incidence of serum ALT elevation >3 × ULN accompanied by a serum bilirubin elevation >2 × ULN. Monitoring of liver tests in clinical practice has shown unconvincing efficacy, but where a benefit-risk analysis would favour continued therapy, monthly monitoring may have some benefit compared with no monitoring at all.
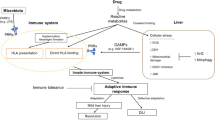
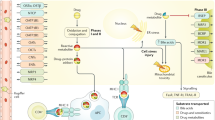
With rare exception, treatment of drug-induced liver injury is principally supportive. Drug toxicity is the most common cause of acute liver failure, defined as a prolonged prothrombin time (international normalised ratio ≥1.5) and any degree of mental alteration occurring <26 weeks after the onset of illness in a patient without pre-existing cirrhosis. A patient who meets these criteria must be evaluated for liver transplantation. The pathogenesis of drug-induced liver injury can be examined on the basis of the two principal patterns of injury. The hepatocellular pattern is characterised by a predominant rise in the level of transaminases and results from the demise of hepatocytes by means of either apoptosis or necrosis. The cholestatic pattern is characterised by a predominant rise of the serum alkaline phosphatase level and usually results from injury to the bile ductular cells either directly by the drug or its metabolite, or indirectly by an adaptive immune response.







Similar content being viewed by others
References
Zimmerman H. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. Philadelphia (PA): Lippincott Williams & Wilkins, 1999
Temple RJ, Himmel MH. Safety of newly approved drugs: implications for prescribing. JAMA 2002; 287: 2273–5
Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 947–54
Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364–72
Watkins PB, Seeff LB. Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology 2006; 43: 618–31
Sgro C, Clinard F, Ouazir K, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology 2002; 36: 451–5
Meier Y, Cavallaro M, Roos M, et al. Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol 2005; 61: 135–43
Kaplowitz N, DeLeve LD. Drug-induced liver disease. New York: Marcel Dekker, 2003
Beaune PH, Lecoeur S. Immunotoxicology of the liver: adverse reactions to drugs. J Hepatol 1997; 26Suppl. 2: 37–42
Neuberger J, Williams R. Immune mechanisms in tienilic acid associated hepatotoxicity. Gut 1989; 30: 515–9
Robin MA, Le Roy M, Descatoire V, et al. Plasma membrane cytochromes P450 as neoantigens and autoimmune targets in drug-induced hepatitis. J Hepatol 1997; 26Suppl. 1: 23–30
Neuberger J. Immune mechanisms in drug hepatotoxicity. Clin Liver Dis 1998; 2: 471–82
Maria VA, Victorino RM. Diagnostic value of specific T cell reactivity to drugs in 95 cases of drug induced liver injury. Gut 1997; 41: 534–40
Maria VA, Victorino RM. Immunological investigation in hepatic drug reactions. Clin Exp Allergy 1998; 28Suppl. 4: 71–7
Davern TJ 2nd, James LP, Hinson JA, et al. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology 2006; 130: 687–94
James LP, Alonso EM, Hynan LS, et al. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics 2006; 118: e676–81
Fleming CR. Hepatobiliary complications in adults receiving nutrition support. Dig Dis 1994; 12: 191–8
Quigley EM, Marsh MN, Shaffer JL, et al. Hepatobiliary complications of total parenteral nutrition. Gastroenterology 1993; 104: 286–301
Benichou C. Criteria of drug-induced liver disorders: report of an international consensus meeting. J Hepatol 1990; 11: 272–6
Navarro V. Hepatic adverse event nomenclature document [online]. Available from URL: http://www.fda.gov/cder/livertox/presentations2005/Vic_Navarro.ppt [Accessed 2006 Nov 17]
Zimmerman H. Drug-induced liver disease. In: Schiff E, ed. Schiff’s diseases of the liver. Baltimore (MD): Lippincott-Raven Publishers, 1999: 973–1064
Bjornsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology 2005; 42: 481–9
Andrade RJ, Lucena MI, Fernandez MC, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology 2005; 129: 512–21
Davidson CS, Leevy CM, Chamberlayne EC, eds. Guidelines for detection of hepatotoxicity due to drugs and chemicals. Fogarthy Conference, 1978. Washington, DC: National Institute of Health, 1979. NIH publication no.: 79–313
Degott C, Feldmann G, Larrey D, et al. Drug-induced prolonged cholestasis in adults: a histological semiquantitative study demonstrating progressive ductopenia. Hepatology 1992; 15: 244–51
Desmet VJ. Vanishing bile duct syndrome in drug-induced liver disease. J Hepatol 1997; 26Suppl. 1: 31–5
Zimmerman HJ. Drug-induced liver disease. Clin Liver Dis 2000; 4: 73–96, vi
Polson J, Lee WM. AASLD position paper: the management of acute liver failure. Hepatology 2005; 41: 1179–97
Carl L. Bilirubin metabolism and the pathophysiology of jaundice. In: Schiff E, ed. Schiff’s diseases of the liver. Philadelphia (PA): Lippincott Williams & Wilkins, 1999
Rotger M, Taffe P, Bleiber G, et al. Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis 2005; 192: 1381–6
Kaplowitz N. Drug-induced liver injury. Clin Infect Dis 2004; 38Suppl. 2: S44–8
Pham T-V. Acetaminophen hepatotoxicity. In: Taylor M, ed. Gastrointestinal emergencies. Baltimore (MD): Williams & Wilkins, 1997: 371–88
Kaplowitz N. Drug-induced liver disorders: implications for drug development and regulation. Drug Saf 2001; 24: 483–90
Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat Rev Drug Discov 2005; 4: 489–99
Gunawan B, Kaplowitz N. Clinical perspectives on xenobiotic-induced hepatotoxicity. Drug Metab Rev 2004; 36: 301–12
Fountain FF, Tolley E, Chrisman CR, et al. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest 2005; 128: 116–23
Maddrey WC. Drug-induced hepatotoxicity: 2005. J Clin Gastroenterol 2005; 39: S83–9
Lee WM. Acute liver failure: etiologies and outcomes. In: Liver diseases: mechanisms of liver injury in emerging therapeutics. AASLD Postgraduate Course 2006. Boston (MA): John B. Hynes Veterans Memorial Convention Center, 2006
Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest 1991; 99: 465–71
Ungo JR, Jones D, Ashkin D, et al. Antituberculosis drug-induced hepatotoxicity: the role of hepatitis C virus and the human immunodeficiency virus. Am J Respir Crit Care Med 1998; 157: 1871–6
Wong WM, Wu PC, Yuen MF, et al. Antituberculosis drug-related liver dysfunction in chronic hepatitis B infection. Hepatology 2000; 31: 201–6
Wu JC, Lee SD, Yeh PF, et al. Isoniazid-rifampin-induced hepatitis in hepatitis B carriers. Gastroenterology 1990; 98: 502–4
Whitcomb DC, Block GD. Association of acetaminophen hepatotoxicity with fasting and ethanol use. JAMA 1994; 272: 1845–50
Zimmerman HJ, Maddrey WC. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: analysis of instances of therapeutic misadventure. Hepatology 1995; 22: 767–73
Bromer MQ, Black M. Acetaminophen hepatotoxicity. Clin Liver Dis 2003; 7: 351–67
Rumack BH. Acetaminophen misconceptions. Hepatology 2004; 40: 10–5
Larrey D, Pageaux GP. Genetic predisposition to drug-induced hepatotoxicity. J Hepatol 1997; 26Suppl. 2: 12–21
Huang YS, Chern HD, Su WJ, et al. Polymorphism of the Nacetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology 2002; 35: 883–9
Huang YS, Chern HD, Su WJ, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology 2003; 37: 924–30
Watanabe I, Tomita A, Shimizu M, et al. A study to survey susceptible genetic factors responsible for troglitazone-associated hepatotoxicity in Japanese patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2003; 73: 435–55
Simon T, Becquemont L, Mary-Krause M, et al. Combined glutathione-S-transferase M1 and T1 genetic polymorphism and tacrine hepatotoxicity. Clin Pharmacol Ther 2000; 67: 432–7
Aithal GP, Ramsay L, Daly AK, et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 2004; 39: 1430–40
Andrade RJ, Lucena MI, Alonso A, et al. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology 2004; 39: 1603–12
Danan G, Benichou C. Causality assessment of adverse reactions to drugs: I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol 1993; 46: 1323–30
Benichou C, Danan G, Flahault A. Causality assessment of adverse reactions to drugs: II. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol 1993; 46: 1331–6
Lucena MI, Camargo R, Andrade RJ, et al. Comparison of two clinical scales for causality assessment in hepatotoxicity. Hepatology 2001; 33: 123–30
Senior J. Regulatory perspectives. In: Kaplowitz N, ed. Drug-induced liver diseases. New York: Marcel Dekker, 2003
Tolman KG. Defining patient risks from expanded preventive therapies. Am J Cardiol 2000; 85: 15–19E
Kaplowitz N. Rules and laws of drug hepatotoxicity. Pharmacoepidemiol Drug Saf 2006; 15: 231–3
Watkins PB, Whitcomb RW. Hepatic dysfunction associated with troglitazone. N Engl J Med 1998; 338: 916–7
Lee WM, Larrey D, Olsson R, et al. Hepatic findings in long-term clinical trials of ximelagatran. Drug Saf 2005; 28: 351–70
Graham DJ, Green L, Senior JR, et al. Troglitazone-induced liver failure: a case study. Am J Med 2003; 114: 299–306
Nolan CM, Goldberg SV, Buskin SE. Hepatotoxicity associated with isoniazid preventive therapy: a 7-year survey from a public health tuberculosis clinic. JAMA 1999; 281: 1014–8
Bohan TP, Helton E, McDonald I, et al. Effect of L-carnitine treatment for valproate-induced hepatotoxicity. Neurology 2001; 56: 1405–9
Rakela J, Mosley JW, Edwards VM, et al. A double-blinded, randomized trial of hydrocortisone in acute hepatic failure. The Acute Hepatic Failure Study Group. Dig Dis Sci 1991; 36: 1223–8
Spagnuolo MI, Iorio R, Vegnente A, et al. Ursodeoxycholic acid for treatment of cholestasis in children on long-term total parenteral nutrition: a pilot study. Gastroenterology 1996; 111: 716–9
Nathwani RA, Kaplowitz N. Drug hepatotoxicity. Clin Liver Dis 2006; 10: 207–17
Walgren JL, Mitchell MD, Thompson DC. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit Rev Toxicol 2005; 35: 325–61
Leist M, Single B, Castoldi AF, et al. Intracellular adenosine triphosphate (ATP) concentration: a switch in the decision between apoptosis and necrosis. J Exp Med 1997; 185: 1481–6
Kaplowitz N. Mechanisms of liver cell injury. J Hepatol 2000; 32: 39–47
Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 1998; 281: 1312–6
Zhao Y, Li S, Childs EE, et al. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J Biol Chem 2001; 276: 27432–40
Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001; 292: 727–30
Puthalakath H, Villunger A, O’Reilly LA, et al. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science 2001; 293: 1829–32
Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998; 281: 1305–8
Faubion WA, Gores GJ. Death receptors in liver biology and pathobiology. Hepatology 1999; 29: 1–4
Czaja MJ, Xu J, Alt E. Prevention of carbon tetrachloride-induced rat liver injury by soluble tumor necrosis factor receptor. Gastroenterology 1995; 108: 1849–54
Morio LA, Chiu H, Sprowles KA, et al. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol 2001; 172: 44–51
Blazka ME, Wilmer JL, Holladay SD, et al. Role of proinflammatory cytokines in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 1995; 133: 43–52
Blazka ME, Elwell MR, Holladay SD, et al. Histopathology of acetaminophen-induced liver changes: role of interleukin 1 alpha and tumor necrosis factor alpha. Toxicol Pathol 1996; 24: 181–9
Boess F, Bopst M, Althaus R, et al. Acetaminophen hepatotoxicity in tumor necrosis factor/lymphotoxin-alpha gene knockout mice. Hepatology 1998; 27: 1021–9
Hogaboam CM, Bone-Larson CL, Steinhauser ML, et al. Exaggerated hepatic injury due to acetaminophen challenge in mice lacking C-C chemokine receptor 2. Am J Pathol 2000; 156: 1245–52
Iimuro Y, Nishiura T, Hellerbrand C, et al. NFkappaB prevents apoptosis and liver dysfunction during liver regeneration. J Clin Invest 1998; 101: 802–11
Leist M, Gantner F, Naumann H, et al. Tumor necrosis factor-induced apoptosis during the poisoning of mice with hepatotoxins. Gastroenterology 1997; 112: 923–34
Colell A, Garcia-Ruiz C, Miranda M, et al. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology 1998; 115: 1541–51
Colell A, Coll O, Garcia-Ruiz C, et al. Tauroursodeoxycholic acid protects hepatocytes from ethanol-fed rats against tumor necrosis factor-induced cell death by replenishing mitochondrial glutathione. Hepatology 2001; 34: 964–71
Ginn-Pease ME, Whisler RL. Optimal NF kappa B mediated transcriptional responses in Jurkat T cells exposed to oxidative stress are dependent on intracellular glutathione and costimulatory signals. Biochem Biophys Res Commun 1996; 226: 695–702
Wiltrout RH. Regulation and antimetastatic functions of liver-associated natural killer cells. Immunol Rev 2000; 174: 63–76 293
Hashimoto W, Takeda K, Anzai R, et al. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol 1995; 154: 4333–40
Doherty DG, O’Farrelly C. Innate and adaptive lymphoid cells in the human liver. Immunol Rev 2000; 174: 5–20
Liu ZX, Govindarajan S, Okamoto S, et al. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J Immunol 2000; 164: 6480–6
Kakimi K, Guidotti LG, Koezuka Y, et al. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med 2000; 192: 921–30
Takeda K, Hayakawa Y, Van Kaer L, et al. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A 2000; 97: 5498–503
Liu ZX, Govindarajan S, Kaplowitz N. Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 2004; 127: 1760–74
Ishida Y, Kondo T, Ohshima T, et al. A pivotal involvement of IFN-gamma in the pathogenesis of acetaminophen-induced acute liver injury. Faseb J 2002; 16: 1227–36
Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 2000; 287: 860–4
Pollack SB, Linnemeyer PA, Gill S. Induction of osteopontin mRNA expression during activation of murine NK cells. J Leukoc Biol 1994; 55: 398–400
Diao H, Kon S, Iwabuchi K, et al. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity 2004; 21: 539–50
Welch KD, Reilly TP, Bourdi M, et al. Genomic identification of potential risk factors during acetaminophen-induced liver disease in susceptible and resistant strains of mice. Chem Res Toxicol 2006; 19: 223–33
Smith GS, Nadig DE, Kokoska ER, et al. Role of neutrophils in hepatotoxicity induced by oral acetaminophen administration in rats. J Surg Res 1998; 80: 252–8
Lawson JA, Farhood A, Hopper RD, et al. The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci 2000; 54: 509–16
Liu ZX, Han D, Gunawan B, et al. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006; 43: 1220–30
Ishida Y, Kondo T, Kimura A, et al. Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol 2006; 36: 1028–38
Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 2006; 2: 493–503
Knowles SR, Uetrecht J, Shear NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet 2000; 356: 1587–91
Uetrecht JP. New concepts in immunology relevant to idiosyncratic drug reactions: the “danger hypothesis” and innate immune system. Chem Res Toxicol 1999; 12: 387–95
Park BK, Pirmohamed M, Kitteringham NR. Role of drug disposition in drug hypersensitivity: a chemical, molecular, and clinical perspective. Chem Res Toxicol 1998; 11: 969–88
Njoku DB, Greenberg RS, Bourdi M, et al. Autoantibodies associated with volatile anesthetic hepatitis found in the sera of a large cohort of pediatric anesthesiologists. Anesth Analg 2002; 94: 243–9
Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol 1994; 12: 991–1045
Gordin FM, Simon GL, Wofsy CB, et al. Adverse reactions to trimethoprim-sulfamethoxazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med 1984; 100: 495–9
Matsumaru K, Ji C, Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology 2003; 37: 1425–34
Acehan D, Jiang X, Morgan DG, et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell 2002; 9: 423–32
Chauhan D, Hideshima T, Rosen S, et al. Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma (MM) cells. J Biol Chem 2001; 276: 24453–6
Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999; 397: 441–6
Lorenzo HK, Susin SA, Penninger J, et al. Apoptosis inducing factor (AIF): a phylogenetically old, caspase-independent effector of cell death. Cell Death Differ 1999; 6: 516–24
Kon K, Kim JS, Jaeschke H, et al. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 2004; 40: 1170–9
Stieger B, Fattinger K, Madon J, et al. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology 2000; 118: 422–30
Bolder U, Trang NV, Hagey LR, et al. Sulindac is excreted into bile by a canalicular bile salt pump and undergoes a cholehepatic circulation in rats. Gastroenterology 1999; 117: 962–71
Fattinger K, Funk C, Pantze M, et al. The endothelin antagonist bosentan inhibits the canalicular bile salt export pump: a potential mechanism for hepatic adverse reactions. Clin Pharmacol Ther 2001; 69: 223–31
Funk C, Ponelle C, Scheuermann G, et al. Cholestatic potential of troglitazone as a possible factor contributing to troglitazone-induced hepatotoxicity: in vivo and in vitro interaction at the canalicular bile salt export pump (Bsep) in the rat. Mol Pharmacol 2001; 59: 627–35
Dietrich CG, Ottenhoff R, de Waart DR, et al. Role of MRP2 and GSH in intrahepatic cycling of toxins. Toxicology 2001; 167: 73–81
Lakehal F, Dansette PM, Becquemont L, et al. Indirect cytotoxicity of flucloxacillin toward human biliary epithelium via metabolite formation in hepatocytes. Chem Res Toxicol 2001; 14: 694–701
Iverson SL, Uetrecht JP. Identification of a reactive metabolite of terbinafine: insights into terbinafine-induced hepatotoxicity. Chem Res Toxicol 2001; 14: 175–81
Cushing HW. The life of Sir William Osler. Oxford: The Clarendon Press, 1925: 2v
Clay KD, Hanson JS, Pope SD, et al. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med 2006; 144: 415–20
Osier W, Bean RB. Sir William Osier: aphorisms from his bedside teachings and writings. Springfield (IL): CC Thomas, 1961: 164
Acknowledgements
No sources of funding were used to assist in the preparation of this article. The authors have no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abboud, G., Kaplowitz, N. Drug-Induced Liver Injury. Drug-Safety 30, 277–294 (2007). https://doi.org/10.2165/00002018-200730040-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200730040-00001