Published online Jan 28, 2006. doi: 10.3748/wjg.v12.i4.526
Revised: August 8, 2005
Accepted: August 25, 2005
Published online: January 28, 2006
Patients with cirrhosis and portal hypertension exhibit characteristic cardiovascular and pulmonary hemodynamic changes. A vasodilatatory state and a hyperdynamic circulation affecting the cardiac and pulmonary functions dominate the circulation. The recently defined cirrhotic cardiomyopathy may affect systolic and diastolic functions, and imply electromechanical abnormalities. In addition, the baroreceptor function and regulation of the circulatory homoeostasis is impaired. Pulmonary dysfunction involves diffusing abnormalities with the development of the hepatopulmonary syndrome and portopulmonary hypertension in some patients. Recent research has focused on the assertion that the hemodynamic and neurohumoral dysregulation are of major importance for the development of the cardiovascular and pulmonary complications in cirrhosis. This aspect is important to take into account in the management of these patients.
- Citation: Møller S, Henriksen JH. Cardiopulmonary complications in chronic liver disease. World J Gastroenterol 2006; 12(4): 526-538
- URL: https://www.wjgnet.com/1007-9327/full/v12/i4/526.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i4.526
The clinical picture of patients with cirrhosis is dominated by the classical complications to portal hypertension, such as ascites, bleeding from esophageal varices, and encephalopathy. In addition, a considerable number of patients show signs of peripheral vasodilatation with palmar erythema and reddish skin, raised and bounding pulse, and a low systemic blood pressure indicating a hyperdynamic circulation[1]. The hyperdynamic syndrome comprises an increased heart rate, cardiac output, and plasma volume, and a reduced systemic vascular resistance and arterial blood pressure[2-6]. The typical circulatory changes in patients with cirrhosis are summarized in Table 1. Increased cardiac output in cirrhosis was described more than 50 years ago[7] and a hyperdynamic, hyporeactive circulation is today a well-characterized element in the clinical appearance of these patients[8]. In addition, patients with cirrhosis develop complications from a variety of organs including the heart, lungs, and kidneys, and other organ systems. Besides the hepatorenal syndrome, this has led to the introduction of new clinical entities, such as cirrhotic cardiomyopathy and the hepatopulmonary syndrome.
| Systemic circulation |
| Plasma volume ↑ |
| Total blood volume ↑ |
| Non-central blood volume ↑ |
| Central and arterial blood volume →↓(↑) |
| Cardiac output (→)↑ |
| Arterial blood pressure →↓ |
| Heart rate ↑ |
| Systemic vascular resistance ↓ |
| Heart |
| Left atrial volume ↑ |
| Left ventricular volume →(↓) |
| Right atrial volume→↑↓ |
| Right ventricular volume →↑↓ |
| Right atrial pressure →↑ |
| Right ventricular end-diastolic pressure → |
| Pulmonary artery pressure →↑ |
| Pulmonary capillary wedge pressure → |
| Left ventricular end-diastolic pressure → |
| Pulmonary circulation |
| Pulmonary blood flow ↑ |
| Pulmonary vascular resistance ↓(↑) |
| Renal circulation |
| Renal blood flow ↓ |
| Renal vascular resistance ↑ |
| Cerebral circulation |
| Cerebral blood flow ↓ → |
| Cutaneous and skeletal muscle circulation |
| Cutaneous blood flow →↑ |
| Skeletal muscular blood flow →↑ |
Among the mechanisms involved in the peripheral arterial vasodilatation in cirrhosis, intensive research has focused on the presence of arteriovenous communications, increased blood volume, and potent vasodilating systems such as the NO and endothelin (ET) systems[9-14]. Activation of a number of neurohumoral homoeostatic system, like the renin-angiotensin-aldosterone systems (RAAS), the sympathetic nervous system (SNS), and the hypothalamic/neuropituitary release of vasopressin also seem to play a pivotal role in the circulatory dysfunction in cirrhosis[15-18].
This review will focus on the pathophysiological aspects of the systemic circulatory abnormalities in cirrhosis with emphasis on cardiac and pulmonary dysfunctions.
The cardiac output is primarily determined by the venous return, heart rate, and myocardial contractility, all of which are controlled by the autonomic nervous system. Among the mechanisms that may raise the cardiac output are increased sympathetic nervous activity, vasodilatation (low systemic vascular resistance), increased blood volume, and the presence of arterio-venous communications. It is noteworthy that the majority of these physiological changes are present in cirrhosis and may more or less contribute to raise cardiac output[1,19]. In the early stages of compensated cirrhosis, the presence of a hyperdynamic circulation is often not apparent. But with the progression of the liver disease, there is an overall association between the severity of cirrhosis and the degree of hyperdynamic circulation. Investigations on circulatory changes and reactivity from the upright to the supine position, and vice versa, suggest that the patients are mostly hyperdynamic in the supine position[20,21]. On the other hand, the pressor systems are relatively deactivated in the supine position and it is well documented that sodium-water excretion is higher in the supine position than in the upright position[22].
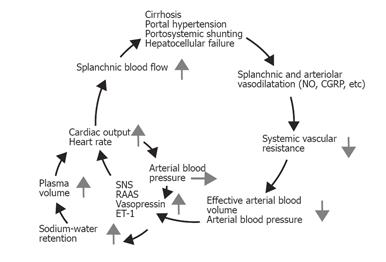
Peripheral vasodilatation in cirrhosis may be brought about either by overproduction of circulating vasodilators, or by vasodilators of intestinal or systemic origin, or by vasodilators that escape degradation in the diseased liver or bypass the liver through portosystemic collaterals[19]. A predominantly splanchnic vasodilation precedes renal sodium and water retention and plasma volume expansion, which correlates with activated counter regulatory vasoconstrictor systems[6,19]. In 1988, Schrier et al proposed the “peripheral arterial vasodilation hypothesis”[23]. According to this theory, primary splanchnic arteriolar vasodilation leads to the reduction of the overall systemic vascular resistance, to avid arterial underfilling with low arterial blood pressure. A reduced effective blood volume, which is that part of the blood volume where baroreceptors are located, leads to the activation of vasoconstrictor systems and secondary sodium-water retention[6,23-25]. Thus, most of the hemodynamic changes seen in cirrhosis can be explained by this theory, as shown in Figure 1.
In recent years, research has focused especially on vasodilating substances, such as NO, calcitonin gene-related peptide (CGRP), and adrenomedullin, but other vasodilators substances, like natriuretic peptides, tumor necrosis factor alpha, interleukins, substance P, ETs, and endocannabinoids, have also been implicated[12,26-32].
NO is synthesized in the vascular endothelium from L-arginine by NO synthase (NOS)[33], of which three isoforms have been identified: inducible NOS (iNOS), constitutive endothelial NOS (ecNOS), and neuronal NOS (ncNOS)[12,34]. In portal hypertension, there seems to be a diminished release of NO from sinusoidal endothelial cells in the cirrhotic liver[34,35] whereas, in the systemic circulation, there is evidence of increased eNOS upregulation, which is probably related to shear stress[26,33,36]. Exhaled air from cirrhotic patients contains higher NO levels than that of controls and correlates with the severity of disease and degree of hyperdynamic circulation; in animal models and cirrhotic patients, blockade of NO formation significantly increases arterial blood pressure and decreases plasma volume and sodium retention[37-40]. Taken together, there is a growing body of evidence that the systemic NO production is increased and precedes the development of the hyperdynamic circulation in cirrhosis, thereby playing a major role in the arteriolar and splanchnic vasodilation and vascular hyporeactivity[12,41]. In addition, vascular endothelial growth factor (VEGF) seems to stimulate angiogenesis and the development of portosystemic collaterals, and recently blockade of the VEGF receptor-2 has been shown to inhibit this process[42]. In spite of the experimental nature of the study, this principle may have some therapeutic implications in the treatment of portal hypertension.
CGRP, a 37-amino-acid neuropeptide with a neurotransmitter function, is on a molar basis the most powerful vasodilating peptide known[43]. It is elevated in cirrhosis, especially in those patients with ascites and the hepatorenal syndrome[43,44] and correlates to hemodynamic markers of vasodilatation and central hypovolaemia, such as cardiac output, systemic vascular resistance, arterial compliance, and central blood volume[27,45-47]. Adrenomedullin is a vasodilating peptide with a sequence similarity to CGRP, it is primarily released from the adrenal medulla and induces relaxation of smooth muscle cells[48]. The circulating levels of adrenomedullin seem to be higher in decompensated patients with cirrhosis and correlate with pressor substances, such as endothelin, renin, vasopressin, and catecholamines[29,49,50].
In addition to a surplus of vasodilators, resistance to pressor hormones may play a role in the pathogenesis of vasodilatation, as patients with cirrhosis are hyporesponsive to the pressor effects of such potent vasopressors as noradrenaline, angiotensin II, and vasopressin[51-53]. This may be brought about by a change in receptor affinity, a decrease in the numbers of receptors, and a variety of post-receptor defects[54,55]. Helmy et al have reported hyporesponsiveness to angiotensin II and endothelin-1, chiefly because of enhanced NO generation[41,56]. Thus, the excess of vasodilators combined with an inadequate hemodynamic response to vasoconstrictors may explain the vasodilatatory state and vascular hyporeactivity in cirrhosis.
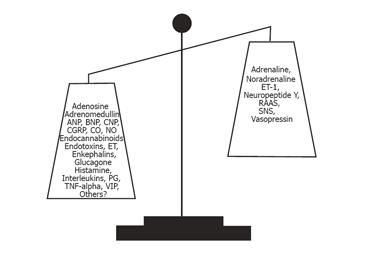
Although cardiac output is increased, thus reflecting substantial vasodilatation, it may cover perfusions from vascular beds that are hyperperfused, normoperfused, and hypoperfused. The kidney in cirrhosis is a vascular region where vasoconstriction prevails and plays a pivotal role in the development of hepatic nephropathy with a decreased renal blood flow and glomerular filtration rate, increased sodium reabsorption, and decreased free water excretion[6,57]. Liver dysfunction, central hypovolaemia, arterial hypotension, and neurohumoral activation with renal vasoconstriction seem to be of major importance. Recent results suggest that a decrease in the cardiac output in the face of severe vasodilatation and activation of the RAAS is an important determinant of the hepatorenal syndrome[58]. Splanchnic vasodilation and central hypovolaemia activate the SNS, the RAAS, the endothelin system, and increased release of vasopressin[17,18]. The imbalance between vasoconstricting and vasodilating forces is illustrated in Figure 2. Secondary hyperaldosteronism and increased tubular sensitivity to aldosterone increase sodium reabsorption in the distal nephron, whereas SNS stimulates sodium reabsorption in proximal tubules, the loop of Henle, and distal tubules[59]. Angiotensin II mainly acts on the efferent arteriole and a low dose of an ACE-inhibitor may induce a significant reduction in glomerular filtration and a further reduction in sodium excretion, even in the absence of a change in arterial blood pressure. This suggests that the integrity of the RAAS is important for the maintenance of renal function in cirrhotic patients, and that RAAS overactivity does not solely contribute to the adverse renal vasoconstriction. Because of the arterial vasodilation, the renal perfusion pressure is low and critically dependent on counter regulatory systems. For these reasons, blockers of these systems by ACE-inhibitors (captopril), angiotensin II antagonists (Losartan), β-adrenergic blockers, and V1 vasopressin antagonist may decrease further the renal blood flow [60,61]. The hepatorenal syndrome denotes a functional and reversible impairment of renal function with a poor prognosis in patients with severe cirrhosis. Treatment is directed towards improving liver function, arterial hypotension, and central hypovolaemia, and reducing renal vasoconstriction for instance with the combined use of splanchnic vasoconstrictors such as terlipressin and plasma expanders like human albumin[62].
Blood and plasma volumes are increased in patients with advanced cirrhosis[25,63], and the distribution of blood in the different vascular beds is abnormal and relates to the severity of the disease[64]. By different techniques it has been established that the central and arterial blood volume is most often decreased, whereas the non-central blood volume, in particular the splanchnic blood volume is increased in animals and patients with cirrhosis[65-67]. The effective arterial blood volume is decreased with relation to the systemic circulatory derangement. Moreover, the central circulation time (i.e. central blood volume relative to CO) is substantially reduced and has a significant relation to poorer survival in advanced cirrhosis[68,69].
During volume expansion, most cirrhotic patients respond with a further reduction in systemic vascular resistance rather than an increase in arterial blood pressure[70,71]. Volume expansion by head-out water immersion provides, in principle, the same volume changes by central relocation as in healthy subjects[70]. However, especially in decompensated cirrhosis, there may be a further decrease in the arterial blood pressure, owing to the unloading of baroreceptors, and renal salt-water excretion is prolonged and incomplete. The infusion of hyperosmotic solutions or albumin in cirrhosis results initially in a shift of fluid from the interstitial space into the plasma volume, with an expansion of the latter[63,71]. Albumin infusion is important in the prevention of postparacentesis circulating dysfunction[72]. When considering volume expansion in terms of the severity of the disease, certain differences become clear. Irrespective of severity, volume expansion produces a rise in stroke volume and cardiac output. Whereas in early cirrhosis there is a proportional expansion of the central and non-central parts of the blood volume, in late cirrhosis expansion is mainly confined to the non-central part, with a proportionally smaller increase in cardiac output, probably because of cardiac dysfunction (cirrhotic cardiomyopathy) and abnormal vascular compliance[71,73].
The increased plasma volume in cirrhosis should be considered secondary to the activation of neurohumoral mechanisms consequent on arterial vasodilatation, low arterial blood pressure, and reduced central and arterial blood volume. However, a non-volume-dependent activation of the SNS through hepatic reflexes, owing to portal hypertension, may occur. This has been documented in animal experiments and there are indications of such a reflex in men[74]. Although the relative importance of non-volume-dependent sympathetic activation and volume/arterial pressure-dependent activation of SNS and other neurohumoral systems has not been finally established, the latter is probably far the most important.
The hyperdynamic circulation in cirrhosis comprises increased cardiac output and work[5,75]. In other circumstances, this would cause cardiac failure, but because of the decreased afterload as reflected by reduced systemic vascular resistance and increased arterial compliance, a left ventricular failure may be latent in cirrhosis[75,76]. Cardiac failure may become manifest under strain or treatment with vasoconstrictors. This type of cardiac dysfunction has been termed as cirrhotic cardiomyopathy and includes impaired cardiac contractility with a systolic dysfunction, diastolic dysfunction, and electromechanical abnormalities with a prolonged Q-T interval[77]. Various electrophysiological mechanisms for the conductance abnormalities and impaired cardiac contractility have been put forward, including reduced beta-adrenoceptor density, post-receptor signal defects, abnormal excitation-contraction coupling, and molecular abnormalities[73].
At rest, when the systemic vascular resistance (afterload) against which the heart works is reduced, cardiac pressures are almost normal and may thereby mask an underlying ventricular dysfunction. Cardiac failure, therefore, becomes manifest only under conditions of hemodynamic stress. Thus, after the exercise, the left ventricular end-diastolic pressure increases, but the expected increase in cardiac stroke index and left ventricular ejection fraction (LVEF) are absent or subnormal, which indicates inadequate ventricular reserve response to a rise in ventricular filling pressure[78,79] A vasoconstrictor-induced an increase in left ventricular afterload by 30% results in an increase in pulmonary capillary wedged pressure about double, without any change in the cardiac output[80]. A similar pattern is seen after the insertion of transjugular intrahepatic portosystemic shunt (TIPS), but the increased cardiac pressures tend to normalize with time[81-83]. A failure to increase cardiac output, despite an increased ventricular filling pressure, indicates that normalization of the afterload impairs cardiac performance and unmasks left ventricular dysfunction[80]. Similar effects are seen after infusion of plasma expanders. Infusion of a plasma protein solution, however, increases cardiac output, as well as the right atrial pressure, pulmonary arterial pressure, and pulmonary capillary wedged pressure, whereas infusion of packed red blood cells may not change these variables[84].
The LVEF, i.e. the stroke volume relative to the left ventricular end-diastolic volume, is an often used measure of systolic function even though it is still very much influenced by preload and afterload. It has been reported to be normal at rest in some studies[78,85-90] and reduced in one study in a subgroup of patients with ascites[91]. The maximum aerobic exercise capacity and maximum heart rate are lower in the majority of patients with cirrhosis[79,85,92]. After exercise, LVEF increases significantly less in cirrhotic patients than in controls[79,92,93]. The reduced functional capacity may be attributed to a combination of blunted heart rate response to exercise, reduced myocardial reserve, and profound skeletal muscle wasting with impaired oxygen extraction[85,94]. Normalization of the low systemic vascular resistance by vasoconstrictors results in an increase in the left atrial and left ventricular filling pressures[75,80]. Therefore, attempts to normalize the reduced cardiac afterload seem to unmask a latent ventricular dysfunction which appears to be resistant to inotropic drugs[77,80]. The expanded blood volume in advanced cirrhosis contributes to a persistent increase in cardiac output, which may overload the heart, with impaired cardiac contractility as the outcome[20,86,95]. In patients with advanced cirrhosis and severe vasodilatation, activation of the RAAS, and impaired renal function, a reduced systolic function (a decrease in cardiac output) seems to be a major determinant for the development of the hepatorenal syndrome[58].
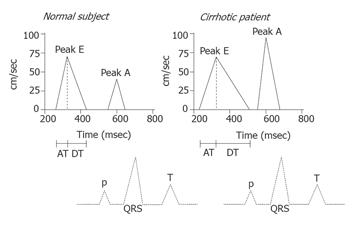
A diastolic dysfunction implies changes in myocardial properties that affect left ventricle acceptance of a sufficient volume during the diastole, despite normal filling pressures; the increased stiffness of the myocardial wall will thus result in impaired filling of the left ventricle[96]. This increases the transmitral pressure gradient and the atrial contribution to ventricular filling in order to normalize the left ventricular diastolic volume. Determinants of a diastolic dysfunction include impaired left ventricular diastolic filling, in spite of high stroke volume. In the Doppler-echocardiogram, the E-wave reflects the early rapid transmitral flow and the A-wave the late atrial contribution to filling. Indications of diastolic dysfunction are a decreased E/A ratio and delayed early diastolic transmitral filling with prolonged deceleration and isovolumetric relaxation times[97], as shown in Figure 3. In cirrhosis, the morphological basis of cirrhotic cardiomyopathy seems to be cardiac hypertrophy, patchy fibrosis, and subendothelial edema[77,93]. In a number of studies, A-wave and E-wave velocities and deceleration times are much increased and the E/A-ratio is decreased in cirrhotic patients, especially in those with ascites[91,97]. Recent studies of ventricular diastolic filling in cirrhosis support the presence of a subclinical myocardial disease with diastolic dysfunction, which, in ascitic patients, is improved after paracentesis and aggravated after TIPS[82,88,91,97]. In these decompensated patients, paracentesis seems to ameliorate diastolic, but not systolic, function[91]. Liver transplantation has recently been shown to revert cardiac alterations, including diastolic dysfunction[93]. It has been proposed that a diastolic dysfunction precedes systolic dysfunction in early heart disease and that antialdosterone treatment improves cardiac function. Pozzi et al recently demonstrated that antialdosterone treatment with K-canrenoate in cirrhosis ameliorated cardiac structure and function, but had almost no effects on systolic and diastolic functions[98]. It is also possible that antialdosterone treatment may have beneficial effects on catecholamine-induced cardiac fibrosis, as described in heart failure[99].
The clinical significance of diastolic dysfunction and the importance in cirrhotic cardiomyopathy have been questioned, as overt cardiac failure is not prominent in cirrhosis. However, there are several reports of unexpected death from heart failure following liver transplantation[100], surgical portocaval shunts and TIPS[81]. These procedures involve a rapid increase in cardiac preload. In a less compliant heart, the diastolic dysfunction could be enough to cause pulmonary edema and heart failure. This is consistent with the findings of Huonker et al[82], who reported an increase in pulmonary artery pressure, pre-load, and diastolic dysfunction after TIPS. Diastolic dysfunction could thus account for part of the cardiac dysfunction in cirrhotic cardiomyopathy.
The sympathetic nervous activity influences the heart rate and electromechanical coupling by several mechanisms: noradrenaline binding to beta-receptors, receptor-mediated G protein interaction, and consequently stimulation of adenylcyclase, activation of cAMP-dependent phosphokinase A, and channel phosphorylation. Several receptor and post-receptor defects have been described in cirrhosis with reduced beta-receptor density and sensitivity[101], and altered G protein and calcium channel functions[102]. All these defects may explain both impaired chronotropic responses and electromechanical uncoupling. This coupling between the cardiac contractions and the arterial system is of major importance to the amount of work performed by the left ventricular myocardium, and thereby, of the strain on the heart[85]. The ascending aorta and aortic arc are the most compliant systemic arteries in the body. The ability to contain the entire stroke volume without excessive deflection in the arterial systolic pressure profile is of crucial importance, especially in patients with a large CO and stroke volume. On the other hand, too compliant a central arterial system will be unable to perform a timely and prompt delivery of blood to the different parts of the body, but may delay the flow to important areas of the vascular bed. Thus, the heart and central arterial tree work together in an essential coordination of the oscillating blood flow. This is especially important when vascular beds with highly different hemodynamic resistances are connected to the central arterial system, as in chronic liver disease.
In addition to the abnormal function of the calcium channels, Ward et al[103] have shown a decrease in K+ currents in ventricular cardiomyocytes from cirrhotic rats, which prolong the Q-T interval. A prolonged Q-T interval is often present in chronic liver disease, potentially leading to ventricular arrhythmias and sudden cardiac death[75,77,104]. Bernardi et al have reported a prolonged Q-T interval, which is significantly related to the severity of the liver disease, plasma noradrenaline, and survival[105]. A reversal of the Q-T interval seems to occur with improved liver function, for instance, after orthotopic liver transplantation[106]. Results from our group indicate that the frequency adjusted Q-TC becomes partly normalized after oral β-blocker treatment[107]. The prolonged Q-T interval in cirrhosis should be considered as an element in the cirrhotic cardiomyopathy and may be of potential use in identifying patients at risk[77]. Pathophysiological and clinical research is needed to assess the prognostic and therapeutic significance of the prolonged Q-TC interval in chronic liver disease.
It can be concluded that there is evidence of a cirrhotic cardiomyopathy in cirrhosis that appears to be unmasked by procedures that stress the heart, such as pharmacological vasoconstriction, exercise, and by such portosystemic shunt procedures as insertion of TIPS. Future studies should be directed against an operable definition of cirrhotic cardiomyopathy, a delineation of the clinical importance, and potential treatment regimens. Until then, we still do not know how or if cirrhotic cardiomyopathy should be treated specifically.
Evidence of autonomic defects in patients with cirrhosis has emerged from hemodynamic responses to standard cardiovascular reflex tests, such as Valsalva ratio, heart rate variability, and isometric exercise[87,108-111]. Most studies of these issues have found a high prevalence of autonomic dysfunction in cirrhosis with associations to liver dysfunction and survival[111,112]. The results of Mohamed et al suggest that the autonomic dysfunction is temporary, arises as a consequence of liver dysfunction, and may be reversible after liver transplantation[106]. Whereas most studies have focused on the defects in the SNS, recent papers have emphasized the importance of a vagal impairment for sodium and fluid retention[108,110,112]. Sympathetic responses to dynamic exercise seem to be normal in patients with cirrhosis, but those to isometric exercise are clearly impaired[113,114]. Similarly, blood pressure responses to orthostasis are impaired, probably because of a blunted baroreflex function[87,115,116]. Abnormal cardiovascular responses to pharmacological stimulations with angiotensin II, noradrenaline, and vasopressin in terms of impaired responses in blood flow and blood pressure have also been reported in cirrhosis[117-119]. Dillon et al[112] have described the correction of autonomic dysfunction in cirrhosis by captopril, which indicates that vagal dysfunction in cirrhosis is partly caused by neuromodulation by angiotensin II. Involvement of the RAAS is also supported by the data from La Villa et al, who recently reported that canrenone, an aldosterone antagonist, normalized cardiac responses to postural changes in compensated cirrhotic patients[116].
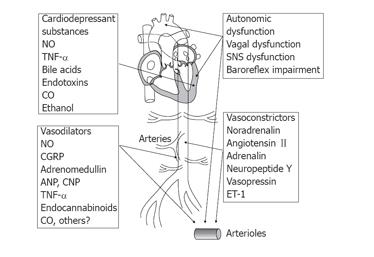
At present the pathophysiological basis of the autonomic dysfunction is unknown, but it could be within the central nervous system through damage to the peripheral nerves or changes in neurotransmission in terms of a post-receptor defect which could explain the vascular hyporeactivity (Figure 4). From the amount of data available, a multifactorial etiology to the hyporesponsiveness in cirrhosis seems most likely.
The hyporeactivity of the vascular system in chronic liver disease is probably a result of a differential balance between vasoconstricting and vasodilating forces in different vascular areas (Figure 2). Generally, however, the vascular system in cirrhosis is very flexible as reflected by an overall increased vascular and arterial compliance[120-122]. The systemic arterial compliance, defined as an increase in intra-arterial volume relative to an increase in transmural arterial blood pressure, is especially increased in patients with decompensated cirrhosis[46]. This is because of the changes in the arterial wall, as well as dynamic changes, and is closely associated with the circulatory and homoeostatic derangement[47,122]. Therefore, the changes in arterial mechanics are partly reversible. The arteriolar tone adjusts the level of blood pressure and may thereby also affect large artery compliance. In fact, arterial compliance depends on the properties of arterial intrinsic elastic and smooth muscle, whereas arteriolar tone should result more from the balance between vasoconstrictors and vasodilators. The increased arterial compliance is directly related to the severity of liver disease and to the circulating vasodilator, CGRP, but is inversely related to circulating adrenaline, and unrelated to indicators of potent vasoconstrictor systems (SNS and ET-1)[47,123]. In addition, other operative elements in the abnormal arterial compliance are blood volume abnormalities, hypoxia, and abnormalities in the C-type natriuretic peptide (CNP), but not to arterial natriuretic peptide. Arterial compliance is not affected by β-adrenergic blockade, but terlipressin almost normalizes it[124]. Arterial compliance is an important determinant of the coupling between the heart and the arterial system, and of the dynamics of intravascular volume relocation[125]. An element in the elevated arterial compliance in advanced cirrhosis is the reduced arterial blood volume and blood pressure[47].
Recent data suggest that the hyperdynamic circulation is mainly caused by circulatory alterations in the splanchnic area[67]. Thus, arteriolar vasodilatation is a more localized event, whereas the increased arterial compliance is more general[47]. Arterial compliance may therefore be an integral variable for vascular responsiveness, together with the systemic vascular resistance. Changed dynamic and static function of the arterial tree may contribute to the abnormal reactions of volume and baroreceptors, and have implications for the abnormal circulatory regulation, and potentially for therapy with vasoactive drugs. These aspects are, however, a topic for further research. In conclusion, arterial compliance is elevated in advanced cirrhosis. Besides a relation to age, body size, gender, and the level of the arterial blood pressure, arterial compliance is directly related to the severity of cirrhosis and the hyperdynamic circulatory derangement.
The arterial vasodilatation contributes to the displacement of the central blood volume towards peripheral and splanchnic vascular regions, resulting in central and arterial hypovolaemia and activated counter regulatory mechanisms[65,71,126]. The pronounced vasodilatation and the fall in arterial blood pressure elicit reduced baroreflex activity and diminished central signaling from the cardioinhibitory center and results primarily in SNS-mediated vasoconstriction of resistance vessels[127]. The normal response to upright posture is a fall in arterial, central venous, and pulse pressures and there is a close relation between the decline in pulse pressure on the one hand and the rising heart rate and declining splanchnic blood flow on the other, which suggests that arterial baroreceptors initiate an increase in heart rate and splanchnic vasoconstriction[127]. Whether this pattern is disturbed in cirrhosis is unclear at present. Some studies have shown that a reduction in thoracic blood volume increases the sensitivity of the arterial baroreflex[127,128]. A resetting of the baroreceptors is still discussed in human conditions in relation to wall tension of the fibroelastic tissues in the vessels and stretch-induced activation of the sodium-potassium channels[127]. As mentioned above, autonomic dysfunction is well-established in cirrhosis[112,114,129], and impaired baroreceptor reflex sensitivity has been suggested[21,115,130]. The baroreceptor function is influenced by hypoxia[131,132] which is often seen in cirrhosis and in particular in those patients with the hepatopulmonary syndrome[133,134].
Baroreceptor sensitivity in the small subset of cirrhotic patients with arterial hypertension has not been investigated, but it is very likely that the co-existence of two different conditions of baroreceptor function involved may affect the cardiovascular regulation[135]. Future studies should seek to reveal the direction of this possible baroreceptor dysfunction in this specific group of patients.
Potent vasodilators such as NO, CGRP, histamine, bradykinin, and serotonin have been implicated in the regulation of the blood pressure in chronic liver disease[1]. A significant inverse relation of the potent vasodilator, adrenomedullin, to arterial blood pressure and ET suggests that these two vasoactive systems play a role in blood pressure regulation in cirrhosis. NOS blockade causes higher arterial blood pressure in cirrhotic rats. Inhibition of the endocannabinoid CB1 receptor raises arterial blood pressure in experimental cirrhosis, and anandamide from the monocytes of cirrhotic rats may contribute to the arterial hypotension observed[32,136]. However, the significance of blood pressure dysregulation in human cirrhosis awaits further studies.
The arterial blood pressure is kept low below normal, depending on the state of the disease, as a circulatory compromise between the vasodilatating and counter regulatory vasoconstricting forces affecting both vascular resistance and compliance. The arterial blood pressure possesses a circadian variation. Twenty-four-hour determinations in cirrhotic patients show that during the day, the systolic, diastolic, and mean arterial blood pressures are substantially reduced, whereas at night, the values are unexpectedly normal[4]. In cirrhosis, the drop from day time to night-time and the rise from night-time to daytime show lower values than in controls. It is known from several diseases, such as uraemia and different types of heart failure, that the circulation of patients classified as “nondippers” is abnormally regulated. The combination of normal blood pressure and increased heart rate at night suggests abnormal regulation of the circulation in cirrhosis. Prolonged rest in the supine position (as during sleep) may lessen the abnormal distribution of the blood volume and improve the ability to maintain a normal “sleeping” arterial blood pressure, only at the cost of an increased heart rate and CO. The upright position further aggravates central hypovolaemia, and normal arterial blood pressure cannot be maintained, even when the heart rate and CO are increased[20,21,137]. The negative correlation of the arterial blood pressure to the Child score during the day and at night confirms that the hemodynamic derangement is related to the severity of the liver disease[69]. The low arterial blood pressure, the abnormal distribution of the circulating medium and diurnal variation in arterial blood pressure, and the marked activation of neurohumoral systems contribute to the abnormal homoeostatic regulation in patients with cirrhosis.
Previous reports of findings that arterial hypertension does not occur together with cirrhosis of the liver are not true in an absolute sense. However, the prevalence of arterial hypertension in cirrhotic patients is substantially reduced, especially in advanced cirrhosis[135]. As hypertensive patients are often effectively treated with diuretics, calcium channel antagonists, beta-blockers, ACE-inhibitors, etc., and some of these drugs are also applied in the treatment of cirrhosis and portal hypertension, the natural history and prevalence of cirrhosis in patients with arterial hypertension, arterial hypertension in patients with cirrhosis, and the interrelationship of these two diseases may be difficult to study today in prospective and untreated cases. Nevertheless, such studies are relevant, since there are many unsolved questions.
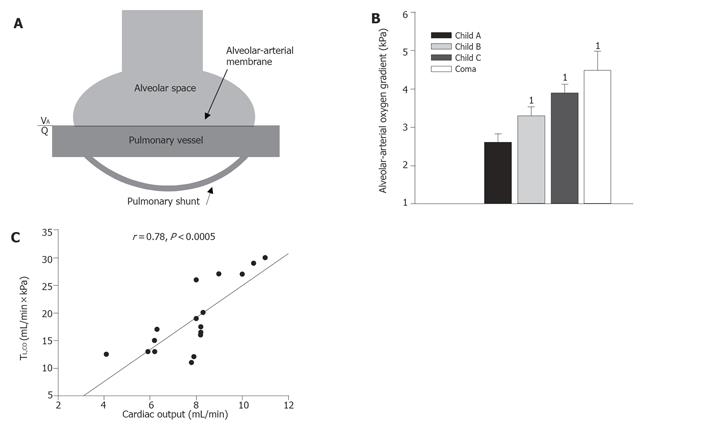
Patients with cirrhosis often complain of dyspnea and platypnea, and arterial oxygenation is often impaired with orthodeoxia[138,139]. The etiology of abnormal lung function and ventilation in cirrhosis may be multifarious and is often a combination of the presence of cardiac dysfunction, heavy smoking, and chronic obstructive lung disease which is common in patients with alcoholic cirrhosis[140]. In addition, lung function and oxygenation can be affected by edema and tense ascites, which are ameliorated after diuretic treatment and paracentesis[141]. But independent of smoking status, patients with cirrhosis have a compromised lung function with a reduced transfer factor and ventilation/perfusion abnormalities[45,134,140,142] and arterial hypoxemia is seen in 30%-70% of patients with chronic liver disease, depending on the severity[143,144]. Various pathophysiological factors may be involved in the reduced diffusing capacity, including an abnormal ventilation/perfusion ratio (VA/Q), the presence of arterial venous shunts, and changes in the alveolar-arterial membrane (Figure 5).
The reduced diffusion capacity (transfer factor) has been related to the increased amount of blood in the lung capillaries[145], but this does not seem to be the case, as there is a direct correlation between the amount of circulating red blood cells and flow in the lung capillaries and the diffusing capacity in normal physiology[146]. To support this concept, we have earlier described direct relations between the diffusing capacity and the cardiac output and central blood volume in cirrhosis (Figure 5)[45].
Pulmonary vascular resistance is most often decreased in cirrhosis[147] and in a substantial number of patients there seem to be areas with a high perfusion rate in relation to alveolar ventilation[45,140]. Besides the abnormal ventilation/perfusion ratio and the presence of regular pulmonary arteriovenous shunts, intrapulmonary vascular dilatations have also been described[134,140,147,148]. The condition with reduced transfer factor, abnormal ventilation/perfusion ratio or shunts, low arterial oxygen saturation, and pulmonary vasodilatation and hyperdynamics is termed as the hepatopulmonary syndrome[134,149,150]. Pulmonary angiography of these patients has revealed two types of patterns with a spongiform appearance of the vessels and small arteriovenous communications[134]. Fallon et al[151-153] have recently reported increased pulmonary vascular endothelial NOS and increased production of cholangiocyte endothelin-1 and increased expression of endothelin-B receptors. Experimental NO-dependent vasodilatation is supported by clinical studies showing increased NO in the exhaled air of cirrhotic patients[154,155]. A recent paper has shown increased carboxyhemoglobin levels that correlate with arterial oxygen tension and alveolar-arterial oxygen gradient in patients with the hepatopulmonary syndrome[156].
The frequency of the hepatopulmonary syndrome in patients with cirrhosis is not yet established. Different reports have given different frequencies of reduced arterial oxygen saturation in cirrhotic patients varying from about 10% to as high as 70%[143,157,158].
The degree of gas exchange abnormalities, such as the oxygen tension and the alveolar-arterial oxygen gradient, correlates with the severity of liver disease (Figure 5). The diagnosis of the hepatopulmonary syndrome relies on the demonstration of arterial hypoxemia (PaO2<9.31 kPa ), an age-adjusted increased alveolar-arterial oxygen gradient (>2.66 kPa) and intrapulmonary vasodilatation[159,160]. According to the severity of deoxygenation, four stages have been proposed[160]. A 100% oxygen shunt study with the patient breathing 100% oxygen may discriminate between functional and anatomic shunts[134]. Contrast-enhanced echocardiography is considered as the method of choice in the diagnosis of the hepatopulmonary syndrome[161]. Agitated saline (microbubbles) is injected into a brachial vein and the bolus is shortly seen in the right heart chambers. A positive test for intrapulmonary vasodilatation occurs with delayed visualization of the microbubbles in the left heart chambers after more than three heart beats[162]. Finally, a lung perfusion scan with the injection of macroaggregated albumin and estimation of the extra-pulmonary shunt fraction can be used[148]. From counts over lungs and brain, the shunt fraction can be calculated and a value >6% is considered positive with a sensitivity of 85%[148,160].
No specific treatment, apart from long-term oxygen therapy, is available for the hepatopulmonary syndrome. It has been reversed by successful orthotopic liver transplantation in some patients[144,163] and by insertion of a TIPS in others[164]. TIPS insertion increases pulmonary artery pressure but cardiorespiratory complications are common and TIPS cannot be recommended for this indication at present[165,166].
The association between portal hypertension and pulmonary artery hypertension is termed portopulmonary hypertension and is defined as a mean pulmonary artery pressure>3.325 kPa and pulmonary vascular resistance >120 dyn∙s/cm5, and normal left atrial pressure (<1.995 kPa)[147]. It is seen infrequently in cirrhosis with an average prevalence from 1% to 4%[167]. Symptoms are typically progressive and include fatigue, dyspnea, and edema[134]. Systemic vascular resistance and cardiac output are not different from that of cirrhotic patients without portopulmonary hypertension, whereas their arterial oxygenation is impaired[168]. The histological appearance of pulmonary vessels is similar to that seen in primary pulmonary artery hypertension, and includes smooth muscle proliferation and hypertrophy[160]. Local vasoconstrictor systems, like the endothelin system, may play a role and recently the administration of a mixed ET-antagonist has showed beneficial effects in portopulmonary hypertension[169,170]. Treatment of portopulmonary hypertension is in general non-specific and palliative, and includes vasodilators, such as calcium channel blockers, nitrates, and prostacyclin[160].
So far, a huge body of research has revealed that, in addition to portal and splanchnic complications to chronic liver disease, complications relating to the systemic and pulmonary circulation affect the prognosis of the patient as part of a multi-organ syndrome. Splanchnic vasodilatation in relation to portal hypertension is responsible for the hyperdynamic circulation and abnormal distribution of blood volume with a reduced “effective arterial blood volume” and activation of baroreceptor and volume-receptor reflexes as the outcome. The enhanced vasodilatation and counter regulatory over-activity of vasoconstrictor systems play major roles in the development of the multi-organ failure in cirrhosis with impaired function and perfusion of kidneys, lungs, brain, skin, and muscles. The function of the heart in cirrhosis is disturbed, with an increased cardiac output and heart rate. Left atrial and ventricular volumes tend to be slightly dilated, whereas the cardiac pressures are normal at rest. Cardiac performance and the systolic and diastolic functions are clearly impaired, in relation to the degree of liver dysfunction. The impaired cardiac contractility, termed cirrhotic cardiomyopathy, is different from that seen in alcoholic heart muscle disease. Reduced β-adrenergic receptor signal transduction and a defective cardiac excitation-contraction coupling are among the significant pathophysiological mechanisms. The cirrhotic heart is overloaded with a high-output failure and at the same time hyperdynamic and dysfunctional, and strain may unmask latent heart failure. In addition, the circulation and the function of a variety of organs are disturbed, including the lungs, kidneys, brain, and peripheral tissues. No specific treatment can be recommended and the only radical treatment option is liver transplantation. A considerable number of patients present with reduced pulmonary vascular resistance, impaired ventilation, and hypoxemia as part of a hepatopulmonary syndrome. A few patients develop portopulmonary hypertension with increased pulmonary vascular resistance.
Although there are still major unsolved questions that remain to be answered, the circulatory and neuroendocrine derangements play important roles in the clinical aggravation, hepatopulmonary dysfunction, and circulatory reactivity. This aspect is important to take into account in the clinical handling of the patient and the assessment of the prognosis.
S- Editor Guo SY and Xu XQ L- Editor Elsevier HK E- Editor Bi L
| 1. | Møller S, Henriksen JH. In: Arroyo V, Gines P, Rodes J, Schrier RW, eds. Ascites and renal dysfunction in liver disease. Malden: Blackwell 1999; 307-329. [Cited in This Article: ] |
| 2. | Llach J, Ginès P, Arroyo V, Rimola A, Titó L, Badalamenti S, Jiménez W, Gaya J, Rivera F, Rodés J. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482-487. [PubMed] [Cited in This Article: ] |
| 3. | Groszmann RJ, Jensen JE. Pathophysiology of portal hypertension. Liver and Biliary Diseases. 2 ed. Baltimore: Williams & Wilkins 1996; 551-562. [Cited in This Article: ] |
| 4. | Møller S, Wiinberg N, Hernriksen JH. Noninvasive 24-hour ambulatory arterial blood pressure monitoring in cirrhosis. Hepatology. 1995;22:88-95. [PubMed] [Cited in This Article: ] |
| 5. | Møller S, Bendtsen F, Henriksen JH. Splanchnic and systemic hemodynamic derangement in decompensated cirrhosis. Can J Gastroenterol. 2001;15:94-106. [PubMed] [Cited in This Article: ] |
| 6. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Kowalski HJ, Abelmann WH, Mcneely WF, Frank NR, Ellis LB. The cardiac output of normal subjects determined by the dye-injection method at rest and during exercise. Am J Med Sci. 1954;228:622-625. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Groszmann RJ. Hyperdynamic circulation of liver disease 40 years later: pathophysiology and clinical consequences. Hepatology. 1994;20:1359-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 208] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide. Lancet. 1991;337:776-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 473] [Cited by in F6Publishing: 460] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 10. | Groszmann RJ. Nitric oxide and hemodynamic impairment. Digestion. 1998;59 Suppl 2:6-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Rockey DC. The cellular pathogenesis of portal hypertension: Stellate cell contractility, endothelin, and nitric oxide. Hepatology. 1997;25:2-5. [Cited in This Article: ] |
| 12. | Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Møller S, Henriksen JH. Endothelins in chronic liver disease. Scand J Clin Lab Invest. 1996;56:481-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Helmy A, Jalan R, Newby DE, Johnston NR, Hayes PC, Webb DJ. Altered peripheral vascular responses to exogenous and endogenous endothelin-1 in patients with well-compensated cirrhosis. Hepatology. 2001;33:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bernardi M, Trevisani F, Gasbarrini A, Gasbarrini G. Hepatorenal disorders: role of the renin-angiotensin-aldosterone system. Semin Liver Dis. 1994;14:23-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Arroyo V, Clària J, Saló J, Jiménez W. Antidiuretic hormone and the pathogenesis of water retention in cirrhosis with ascites. Semin Liver Dis. 1994;14:44-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 59] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Møller S, Henriksen JH. Neurohumoral fluid regulation in chronic liver disease. Scand J Clin Lab Invest. 1998;58:361-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Henriksen JH, Møller S, Ring-Larsen H, Christensen NJ. The sympathetic nervous system in liver disease. J Hepatol. 1998;29:328-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Groszmann RJ. Vasodilatation and hyperdynamic circulatory state in chronic liver disease. Portal hypertension. Pathophysiology and treatment. 1 ed. Oxford: Blackwell 1994; 17-26. [Cited in This Article: ] |
| 20. | Bernardi M, Fornalè L, Di Marco C, Trevisani F, Baraldini M, Gasbarrini A, De Collibus C, Zacà F, Ligabue A, Colantoni A. Hyperdynamic circulation of advanced cirrhosis: a re-appraisal based on posture-induced changes in hemodynamics. J Hepatol. 1995;22:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Laffi G, Lagi A, Cipriani M, Barletta G, Bernardi L, Fattorini L, Melani L, Riccardi D, Bandinelli G, Mannelli M. Impaired cardiovascular autonomic response to passive tilting in cirrhosis with ascites. Hepatology. 1996;24:1063-1067. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Ring-Larsen H, Henriksen JH, Wilken C, Clausen J, Pals H, Christensen NJ. Diuretic treatment in decompensated cirrhosis and congestive heart failure: effect of posture. Br Med J (Clin Res Ed). 1986;292:1351-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1131] [Cited by in F6Publishing: 984] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 24. | Møller S, Henriksen JH. Circulatory abnormalities in cirrhosis with focus on neurohumoral aspects. Semin Nephrol. 1997;17:505-519. [PubMed] [Cited in This Article: ] |
| 25. | Schrier RW, Ecder T. Gibbs memorial lecture. Unifying hypothesis of body fluid volume regulation: implications for cardiac failure and cirrhosis. Mt Sinai J Med. 2001;68:350-361. [PubMed] [Cited in This Article: ] |
| 26. | Martin PY, Ginès P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533-541. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 262] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Møller S, Bendtsen F, Schifter S, Henriksen JH. Relation of calcitonin gene-related peptide to systemic vasodilatation and central hypovolaemia in cirrhosis. Scand J Gastroenterol. 1996;31:928-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Hori N, Okanoue T, Sawa Y, Kashima K. Role of calcitonin gene-related peptide in the vascular system on the development of the hyperdynamic circulation in conscious cirrhotic rats. J Hepatol. 1997;26:1111-1119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Guevara M, Ginès P, Jiménez W, Sort P, Fernández-Esparrach G, Escorsell A, Bataller R, Bosch J, Arroyo V, Rivera F. Increased adrenomedullin levels in cirrhosis: relationship with hemodynamic abnormalities and vasoconstrictor systems. Gastroenterology. 1998;114:336-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A. Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis. Nat Med. 2001;7:827-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 252] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 31. | Lee FY, Lin HC, Tsai YT, Chang FY, Lu RH, Hou MC, Li CP, Chu CJ, Wang SS, Lee SD. Plasma substance P levels in patients with liver cirrhosis: relationship to systemic and portal hemodynamics. Am J Gastroenterol. 1997;92:2080-2084. [PubMed] [Cited in This Article: ] |
| 32. | Ros J, Clària J, To-Figueras J, Planagumà A, Cejudo-Martín P, Fernández-Varo G, Martín-Ruiz R, Arroyo V, Rivera F, Rodés J. Endogenous cannabinoids: a new system involved in the homeostasis of arterial pressure in experimental cirrhosis in the rat. Gastroenterology. 2002;122:85-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Farzaneh-Far R, Moore K. Nitric oxide and the liver. Liver. 2001;21:161-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Rockey DC, Chung JJ. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 261] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci U S A. 2002;99:17161-17166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 128] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 36. | Tazi KA, Barrière E, Moreau R, Heller J, Sogni P, Pateron D, Poirel O, Lebrec D. Role of shear stress in aortic eNOS up-regulation in rats with biliary cirrhosis. Gastroenterology. 2002;122:1869-1877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Churchill MA, Geraci JE, Hunder GG. Musculoskeletal manifestations of bacterial endocarditis. Ann Intern Med. 1977;87:754-759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 143] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 38. | Lee FY, Colombato LA, Albillos A, Groszmann RJ. N omega-nitro-L-arginine administration corrects peripheral vasodilation and systemic capillary hypotension and ameliorates plasma volume expansion and sodium retention in portal hypertensive rats. Hepatology. 1993;17:84-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Niederberger M, Martin PY, Ginès P, Morris K, Tsai P, Xu DL, McMurtry I, Schrier RW. Normalization of nitric oxide production corrects arterial vasodilation and hyperdynamic circulation in cirrhotic rats. Gastroenterology. 1995;109:1624-1630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 132] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 40. | La Villa G, Barletta G, Pantaleo P, Del Bene R, Vizzutti F, Vecchiarino S, Masini E, Perfetto F, Tarquini R, Gentilini P. Hemodynamic, renal, and endocrine effects of acute inhibition of nitric oxide synthase in compensated cirrhosis. Hepatology. 2001;34:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 41. | Helmy A, Newby DE, Jalan R, Johnston NR, Hayes PC, Webb DJ. Nitric oxide mediates the reduced vasoconstrictor response to angiotensin II in patients with preascitic cirrhosis. J Hepatol. 2003;38:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Fernandez M, Mejias M, Angermayr B, Garcia-Pagan JC, Rodés J, Bosch J. Inhibition of VEGF receptor-2 decreases the development of hyperdynamic splanchnic circulation and portal-systemic collateral vessels in portal hypertensive rats. J Hepatol. 2005;43:98-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 146] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Gupta S, Morgan TR, Gordan GS. Calcitonin gene-related peptide in hepatorenal syndrome. A possible mediator of peripheral vasodilation. J Clin Gastroenterol. 1992;14:122-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Bendtsen F, Schifter S, Henriksen JH. Increased circulating calcitonin gene-related peptide (CGRP) in cirrhosis. J Hepatol. 1991;12:118-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Møller S, Becker U, Schifter S, Abrahamsen J, Henriksen JH. Effect of oxygen inhalation on systemic, central, and splanchnic haemodynamics in cirrhosis. J Hepatol. 1996;25:316-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Henriksen JH, Møller S, Schifter S, Bendtsen F. Increased arterial compliance in decompensated cirrhosis. J Hepatol. 1999;31:712-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Henriksen JH, Møller S, Schifter S, Abrahamsen J, Becker U. High arterial compliance in cirrhosis is related to low adrenaline and elevated circulating calcitonin gene related peptide but not to activated vasoconstrictor systems. Gut. 2001;49:112-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Richards AM, Nicholls MG, Lewis L, Lainchbury JG. Adrenomedullin. Clin Sci (Lond). 1996;91:3-16. [PubMed] [Cited in This Article: ] |
| 49. | Fábrega E, Casafont F, Crespo J, de la Peña J, San Miguel G, de las Heras G, García-Unzueta MT, Amado JA, Pons-Romero F. Plasma adrenomedullin levels in patients with hepatic cirrhosis. Am J Gastroenterol. 1997;92:1901-1904. [PubMed] [Cited in This Article: ] |
| 50. | Genesca J, Gonzalez A, Catalan R, Segura R, Martinez M, Esteban R, Groszmann RJ, Guardia J. Adrenomedullin, a vasodilator peptide implicated in hemodynamic alterations of liver cirrhosis: relationship to nitric oxide. Dig Dis Sci. 1999;44:372-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Polio J, Sieber CC, Lerner E, Groszmann RJ. Cardiovascular hyporesponsiveness to norepinephrine, propranolol and nitroglycerin in portal-hypertensive and aged rats. Hepatology. 1993;18:128-136. [PubMed] [Cited in This Article: ] |
| 52. | Karatapanis S, McCormick PA, Kakad S, Chin JK, Islam M, Jeremy J, Harry D, McIntyre N, Burroughs AK, Jacobs M. Alteration in vascular reactivity in isolated aortic rings from portal vein-constricted rats. Hepatology. 1994;20:1516-1521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Newby DE, Jalan R, Masumori S, Hayes PC, Boon NA, Webb DJ. Peripheral vascular tone in patients with cirrhosis: role of the renin-angiotensin and sympathetic nervous systems. Cardiovasc Res. 1998;38:221-228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 54. | Moreau R, Komeichi H, Kirstetter P, Ohsuga M, Cailmail S, Lebrec D. Altered control of vascular tone by adenosine triphosphate-sensitive potassium channels in rats with cirrhosis. Gastroenterology. 1994;106:1016-1023. [PubMed] [Cited in This Article: ] |
| 55. | Jaue DN, Ma Z, Lee SS. Cardiac muscarinic receptor function in rats with cirrhotic cardiomyopathy. Hepatology. 1997;25:1361-1365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 56. | Helmy A, Newby DE, Jalan R, Hayes PC, Webb DJ. Enhanced vasodilatation to endothelin antagonism in patients with compensated cirrhosis and the role of nitric oxide. Gut. 2003;52:410-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 57. | Henriksen JH. Cirrhosis: ascites and hepatorenal syndrome. Recent advances in pathogenesis. J Hepatol. 1995;23 Suppl 1:25-30. [PubMed] [Cited in This Article: ] |
| 58. | Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Ginès P, Moreira V, Milicua JM, Jiménez W, Arroyo V. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 412] [Cited by in F6Publishing: 360] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 59. | DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75-197. [PubMed] [Cited in This Article: ] |
| 60. | Gentilini P, Romanelli RG, La Villa G, Maggiore Q, Pesciullesi E, Cappelli G, Casini Raggi V, Foschi M, Marra F, Pinzani M. Effects of low-dose captopril on renal hemodynamics and function in patients with cirrhosis of the liver. Gastroenterology. 1993;104:588-594. [PubMed] [Cited in This Article: ] |
| 61. | González-Abraldes J, Albillos A, Bañares R, Del Arbol LR, Moitinho E, Rodríguez C, González M, Escorsell A, García-Pagán JC, Bosch J. Randomized comparison of long-term losartan versus propranolol in lowering portal pressure in cirrhosis. Gastroenterology. 2001;121:382-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 120] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Møller S, Henriksen JH. Review article: pathogenesis and pathophysiology of hepatorenal syndrome--is there scope for prevention. Aliment Pharmacol Ther. 2004;20 Suppl 3:31-41; discussion 42-3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Brinch K, Møller S, Bendtsen F, Becker U, Henriksen JH. Plasma volume expansion by albumin in cirrhosis. Relation to blood volume distribution, arterial compliance and severity of disease. J Hepatol. 2003;39:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Møller S, Henriksen JH, Bendtsen F. Central and noncentral blood volumes in cirrhosis: relationship to anthropometrics and gender. Am J Physiol Gastrointest Liver Physiol. 2003;284:G970-G979. [PubMed] [Cited in This Article: ] |
| 65. | Colombato LA, Albillos A, Groszmann RJ. The role of central blood volume in the development of sodium retention in portal hypertensive rats. Gastroenterology. 1996;110:193-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Henriksen JH, Bendtsen F, Sørensen TI, Stadeager C, Ring-Larsen H. Reduced central blood volume in cirrhosis. Gastroenterology. 1989;97:1506-1513. [PubMed] [Cited in This Article: ] |
| 67. | Kiszka-Kanowitz M, Henriksen JH, Møller S, Bendtsen F. Blood volume distribution in patients with cirrhosis: aspects of the dual-head gamma-camera technique. J Hepatol. 2001;35:605-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 193] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Møller S, Bendtsen F, Christensen E, Henriksen JH. Prognostic variables in patients with cirrhosis and oesophageal varices without prior bleeding. J Hepatol. 1994;21:940-946. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Epstein M. Renal sodium handling in liver disease. The Kidney in Liver Disease. 4 ed. Philadelphia: Hanley and Belfus 1996; 1-31. [Cited in This Article: ] |
| 71. | Møller S, Bendtsen F, Henriksen JH. Effect of volume expansion on systemic hemodynamics and central and arterial blood volume in cirrhosis. Gastroenterology. 1995;109:1917-1925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 72. | Ginès P, Arroyo V. Paracentesis in the management of cirrhotic ascites. J Hepatol. 1993;17 Suppl 2:S14-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Møller S, Henriksen JH. Cardiovascular dysfunction in cirrhosis. Pathophysiological evidence of a cirrhotic cardiomyopathy. Scand J Gastroenterol. 2001;36:785-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Jiménez-Sáenz M, Ortiz-Moyano C, Cantillana-Martinez J, Herrerías-Gutierrez JM. Recurrent abdominal pain in systemic sclerosis: not always intestinal pseudo-obstruction. J Eur Acad Dermatol Venereol. 2003;17:605-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 75. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy: a pathophysiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 76. | Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26:842-847. [PubMed] [Cited in This Article: ] |
| 77. | Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 200] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 78. | Kelbaek H, Rabøl A, Brynjolf I, Eriksen J, Bonnevie O, Godtfredsen J, Munck O, Lund JO. Haemodynamic response to exercise in patients with alcoholic liver cirrhosis. Clin Physiol. 1987;7:35-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 79. | Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 80. | Limas CJ, Guiha NH, Lekagul O, Cohn JN. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation. 1974;49:754-760. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 66] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, Gadano A, Lassen C, Benhamou JP, Erlinger S. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25:135-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 328] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 82. | Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rössle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 83. | Merli M, Valeriano V, Funaro S, Attili AF, Masini A, Efrati C, De CS, Riggio O. Modifications of cardiac function in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt (TIPS). Am J Gastroenterol. 2002;97:142-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 84. | Elizalde JI, Moitinho E, García-Pagán JC, Cirera I, Escorsell A, Bandi JC, Jiménez W, Bosch J, Piqué JM, Rodés J. Effects of increasing blood hemoglobin levels on systemic hemodynamics of acutely anemic cirrhotic patients. J Hepatol. 1998;29:789-795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bouchier IA, Hayes PC. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 86. | Møller S, Søndergaard L, Møgelvang J, Henriksen O, Henriksen JH. Decreased right heart blood volume determined by magnetic resonance imaging: evidence of central underfilling in cirrhosis. Hepatology. 1995;22:472-478. [PubMed] [Cited in This Article: ] |
| 87. | Laffi G, Barletta G, La Villa G, Del Bene R, Riccardi D, Ticali P, Melani L, Fantini F, Gentilini P. Altered cardiovascular responsiveness to active tilting in nonalcoholic cirrhosis. Gastroenterology. 1997;113:891-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 88. | Wong F, Liu P, Lilly L, Bomzon A, Blendis L. Role of cardiac structural and functional abnormalities in the pathogenesis of hyperdynamic circulation and renal sodium retention in cirrhosis. Clin Sci (Lond). 1999;97:259-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 89. | Valeriano V, Funaro S, Lionetti R, Riggio O, Pulcinelli G, Fiore P, Masini A, De Castro S, Merli M. Modification of cardiac function in cirrhotic patients with and without ascites. Am J Gastroenterol. 2000;95:3200-3205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 90. | De BK, Majumdar D, Das D, Biswas PK, Mandal SK, Ray S, Bandopadhyay K, Das TK, Dasgupta S, Guru S. Cardiac dysfunction in portal hypertension among patients with cirrhosis and non-cirrhotic portal fibrosis. J Hepatol. 2003;39:315-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 91. | Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131-1137. [PubMed] [Cited in This Article: ] |
| 92. | Kelbaek H, Eriksen J, Brynjolf I, Raboel A, Lund JO, Munck O, Bonnevie O, Godtfredsen J. Cardiac performance in patients with asymptomatic alcoholic cirrhosis of the liver. Am J Cardiol. 1984;54:852-825. [Cited in This Article: ] |
| 93. | Torregrosa M, Aguadé S, Dos L, Segura R, Gónzalez A, Evangelista A, Castell J, Margarit C, Esteban R, Guardia J. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 94. | Epstein SK, Ciubotaru RL, Zilberberg MD, Kaplan LM, Jacoby C, Freeman R, Kaplan MM. Analysis of impaired exercise capacity in patients with cirrhosis. Dig Dis Sci. 1998;43:1701-1707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Friedman HS, Cirillo N, Schiano F, Nathan P, Khan S, Rosero H, Vaseghi M, Sacchi T, Vasavada B, Bjornson L. Vasodilatory state of decompensated cirrhosis: relation to hepatic dysfunction, ascites, and vasoactive substances. Alcohol Clin Exp Res. 1995;19:123-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Opie LH. The Heart. Physiology, from cell to circulation. 3 ed. Philadelphia: Lippincott 1998; . [Cited in This Article: ] |
| 97. | Finucci G, Desideri A, Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996;31:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Pozzi M, Grassi G, Ratti L, Favini G, Dell'Oro R, Redaelli E, Calchera I, Boari G, Mancia G. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. Am J Gastroenterol. 2005;100:1110-1116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Bos R, Mougenot N, Findji L, Médiani O, Vanhoutte PM, Lechat P. Inhibition of catecholamine-induced cardiac fibrosis by an aldosterone antagonist. J Cardiovasc Pharmacol. 2005;45:8-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 100. | Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000;6:S44-S52. [Cited in This Article: ] |
| 101. | Liu H, Song D, Lee SS. Cirrhotic cardiomyopathy. Gastroenterol Clin Biol. 2002;26:842-847. [Cited in This Article: ] |
| 102. | Zavecz JH, Bueno O, Maloney RE, O'Donnell JM, Roerig SC, Battarbee HD. Cardiac excitation-contraction coupling in the portal hypertensive rat. Am J Physiol Gastrointest Liver Physiol. 2000;279:G28-G39. [PubMed] [Cited in This Article: ] |
| 103. | Ward CA, Ma Z, Lee SS, Giles WR. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol. 1997;273:G537-G544. [PubMed] [Cited in This Article: ] |
| 104. | Day CP, James OF, Butler TJ, Campbell RW. QT prolongation and sudden cardiac death in patients with alcoholic liver disease. Lancet. 1993;341:1423-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 182] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 105. | Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, Schepis F, Mandini M, Simoni P, Contin M. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 265] [Cited by in F6Publishing: 236] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 106. | Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 107. | Henriksen JH, Bendtsen F, Hansen EF, Møller S. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004;40:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 108. | Hendrickse MT, Triger DR. Vagal dysfunction and impaired urinary sodium and water excretion in cirrhosis. Am J Gastroenterol. 1994;89:750-757. [PubMed] [Cited in This Article: ] |
| 109. | Oliver MI, Miralles R, Rubíes-Prat J, Navarro X, Espadaler JM, Solá R, Andreu M. Autonomic dysfunction in patients with non-alcoholic chronic liver disease. J Hepatol. 1997;26:1242-1248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Trevisani F, Sica G, Mainquà P, Santese G, De Notariis S, Caraceni P, Domenicali M, Zacà F, Grazi GL, Mazziotti A. Autonomic dysfunction and hyperdynamic circulation in cirrhosis with ascites. Hepatology. 1999;30:1387-1392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Rangari M, Sinha S, Kapoor D, Mohan JC, Sarin SK. Prevalence of autonomic dysfunction in cirrhotic and noncirrhotic portal hypertension. Am J Gastroenterol. 2002;97:707-713. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 112. | Dillon JF, Nolan J, Thomas H, Williams BC, Neilson JM, Bouchier IA, Hayes PC. The correction of autonomic dysfunction in cirrhosis by captopril. J Hepatol. 1997;26:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 113. | Bernardi M, Rubboli A, Trevisani F, Cancellieri C, Ligabue A, Baraldini M, Gasbarrini G. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J Hepatol. 1991;12:207-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 89] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | MacGilchrist AJ, Sumner D, Reid JL. Impaired pressor reactivity in cirrhosis: evidence for a peripheral vascular defect. Hepatology. 1991;13:689-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Arranz CT, Balaszczuk AM, Costa MA, Eizayaga FX, Romay S, Mongelli C, Lemberg A. Systemic baroreflex alterations in prehepatic portal hypertensive conscious rats. Arch Physiol Biochem. 1995;103:422-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | La Villa G, Barletta G, Romanelli RG, Laffi G, Del Bene R, Vizzutti F, Pantaleo P, Mazzocchi V, Gentilini P. Cardiovascular effects of canrenone in patients with preascitic cirrhosis. Hepatology. 2002;35:1441-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 117. | MacGilchrist AJ, Sumner D, Reid JL. Impaired pressor reactivity in cirrhosis: evidence for a peripheral vascular defect. Hepatology. 1991;13:689-694. [Cited in This Article: ] |
| 118. | Moreau R, Hadengue A, Soupison T, Mechin G, Assous M, Roche-Sicot J, Sicot C. Abnormal pressor response to vasopressin in patients with cirrhosis: evidence for impaired buffering mechanisms. Hepatology. 1990;12:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Ryan J, Sudhir K, Jennings G, Esler M, Dudley F. Impaired reactivity of the peripheral vasculature to pressor agents in alcoholic cirrhosis. Gastroenterology. 1993;105:1167-1172. [PubMed] [Cited in This Article: ] |
| 120. | Hadengue A, Moreau R, Gaudin C, Bacq Y, Champigneulle B, Lebrec D. Total effective vascular compliance in patients with cirrhosis: a study of the response to acute blood volume expansion. Hepatology. 1992;15:809-815. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 43] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 121. | Andreu V, Perello A, Moitinho E, Escorsell A, García-Pagán JC, Bosch J, Rodés J. Total effective vascular compliance in patients with cirrhosis. Effects of propranolol. J Hepatol. 2002;36:356-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 122. | Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Møller S. Arterial compliance in patients with cirrhosis: stroke volume-pulse pressure ratio as simplified index. Am J Physiol Gastrointest Liver Physiol. 2001;280:G584-G594. [PubMed] [Cited in This Article: ] |
| 123. | Møller S, Gülberg V, Becker U, Gerbes AL, Henriksen JH. Elevated arterial compliance in patients with cirrhosis is not related to arterial endothelin-1. Scand J Gastroenterol. 2002;37:1064-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 124. | Møller S, Hansen EF, Becker U, Brinch K, Henriksen JH, Bendtsen F. Central and systemic haemodynamic effects of terlipressin in portal hypertensive patients. Liver. 2000;20:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 125. | Rowell LB. Human cardiovascular control. Oxford: Oxford University Press 1993; . [Cited in This Article: ] |
| 126. | Salerno F, Cazzaniga M, Pagnozzi G, Cirello I, Nicolini A, Meregaglia D, Burdick L. Humoral and cardiac effects of TIPS in cirrhotic patients with different "effective" blood volume. Hepatology. 2003;38:1370-1377. [PubMed] [Cited in This Article: ] |
| 127. | Rowell LB. Reflex control during orthostasis. ed. Oxford: Oxford University Press 1993; 37-80. [Cited in This Article: ] |
| 128. | Mancia G, Mark AL. Arterial baroreflexes in humans. Handbook of Physiology. Vol III. The cardiovascular system.Peripheral circulation and organ blood flow. Baltimore: Waverly Press Inc 1983; 755-793. [Cited in This Article: ] |
| 129. | Hendrickse MT, Thuluvath PJ, Triger DR. Natural history of autonomic neuropathy in chronic liver disease. Lancet. 1992;339:1462-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 130. | Veglio F, Melchio R, Calva S, Rabbia F, Gallo V, Molino P, Mengozzi G, Mulatero P, Martini G, Riva P. Noninvasive assessment of spontaneous baroreflex sensitivity in patients with liver cirrhosis. Liver. 1998;18:420-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 131. | Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol (1985). 2002;93:857-864. [PubMed] [Cited in This Article: ] |
| 132. | Ogoh S, Volianitis S, Nissen P, Wray DW, Secher NH, Raven PB. Carotid baroreflex responsiveness to head-up tilt-induced central hypovolaemia: effect of aerobic fitness. J Physiol. 2003;551:601-608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 133. | Krowka MJ. Hepatopulmonary syndrome versus portopulmonary hypertension: distinctions and dilemmas. Hepatology. 1997;25:1282-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 134. | Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859-865. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 163] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 135. | Henriksen JH, Møller S. Hypertension and liver disease. Curr Hypertens Rep. 2004;6:453-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 136. | Fernández-Varo G, Ros J, Morales-Ruiz M, Cejudo-Martín P, Arroyo V, Solé M, Rivera F, Rodés J, Jiménez W. Nitric oxide synthase 3-dependent vascular remodeling and circulatory dysfunction in cirrhosis. Am J Pathol. 2003;162:1985-1993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 137. | Møller S, Nørgaard A, Henriksen JH, Frandsen E, Bendtsen F. Effects of tilting on central hemodynamics and homeostatic mechanisms in cirrhosis. Hepatology. 2004;40:811-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 138. | Agustí AG, Roca J, Bosch J, Rodriguez-Roisin R. The lung in patients with cirrhosis. J Hepatol. 1990;10:251-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 139. | Moreau R, Lee SS, Soupison T, Roche-Sicot J, Sicot C. Abnormal tissue oxygenation in patients with cirrhosis and liver failure. J Hepatol. 1988;7:98-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 95] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 140. | Agusti AG, Roca J, Rodriguez-Roisin R. Mechanisms of gas exchange impairment in patients with liver cirrhosis. Clin Chest Med. 1996;17:49-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 141. | Byrd RP, Roy TM, Simons M. Improvement in oxygenation after large volume paracentesis. South Med J. 1996;89:689-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 142. | Krowka MJ. Hepatopulmonary syndromes. Gut. 2000;46:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 109] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 143. | Møller S, Hillingsø J, Christensen E, Henriksen JH. Arterial hypoxaemia in cirrhosis: fact or fiction. Gut. 1998;42:868-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 144. | Martínez GP, Barberà JA, Visa J, Rimola A, Paré JC, Roca J, Navasa M, Rodés J, Rodriguez-Roisin R. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 162] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 145. | Rakela J, Krowka MJ. Cardiovascular and pulmonary complications of liver disease. Hepatology. A textbook of liver disease. 3 ed. Philadelphia: W.B.Saunders 1996; 675-684. [Cited in This Article: ] |
| 146. | Cotes JE, Chinn DJ, Quanjer PH, Roca J, Yernault JC. [Standardization of the measurement of transfer factor (diffusing capacity). Work Group on Standardization of Respiratory Function Tests. European Community for Coal and Steel. Official position of the European Respiratory Society]. Rev Mal Respir. 1994;11 Suppl 3:41-52. [PubMed] [Cited in This Article: ] |
| 147. | Hervé P, Lebrec D, Brenot F, Simonneau G, Humbert M, Sitbon O, Duroux P. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 251] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 148. | Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 185] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 149. | Rodríguez-Roisin R, Agustí AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 178] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 150. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 151. | Fallon MB, Abrams GA, Luo B, Hou Z, Dai J, Ku DD. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology. 1997;113:606-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 150] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 152. | Luo B, Liu L, Tang L, Zhang J, Stockard CR, Grizzle WE, Fallon MB. Increased pulmonary vascular endothelin B receptor expression and responsiveness to endothelin-1 in cirrhotic and portal hypertensive rats: a potential mechanism in experimental hepatopulmonary syndrome. J Hepatol. 2003;38:556-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 153. | Fallon MB. Mechanisms of pulmonary vascular complications of liver disease: hepatopulmonary syndrome. J Clin Gastroenterol. 2005;39:S138-S142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 154. | Söderman C, Leone A, Furst V, Persson MG. Endogenous nitric oxide in exhaled air from patients with liver cirrhosis. Scand J Gastroenterol. 1997;32:591-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 155. | Rolla G, Brussino L, Colagrande P, Dutto L, Polizzi S, Scappaticci E, Bergerone S, Morello M, Marzano A, Martinasso G. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology. 1997;26:842-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 91] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 156. | Arguedas MR, Drake BB, Kapoor A, Fallon MB. Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome. Gastroenterology. 2005;128:328-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 157. | Rodriguez-Roisin R, Krowka MJ. Is severe arterial hypoxaemia due to hepatic disease an indication for liver transplantation A new therapeutic approach. Eur Respir J. 1994;7:839-842. [PubMed] [Cited in This Article: ] |
| 158. | Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 233] [Cited by in F6Publishing: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 159. | Krowka MJ. Portopulmonary hypertension and the issue of survival. Liver Transpl. 2005;11:1026-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 160. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 600] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 161. | Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283-1288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 273] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 162. | Aller R, Moya JL, Moreira V, García-Lledo A, Sanromán AL, Paino C, Boixeda D. Diagnosis and grading of intrapulmonary vascular dilatation in cirrhotic patients with contrast transesophageal echocardiography. J Hepatol. 1999;31:1044-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 163. | Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 164. | Riegler JL, Lang KA, Johnson SP, Westerman JH. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology. 1995;109:978-983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Schwartz JM, Beymer C, Althaus SJ, Larson AM, Zaman A, Glickerman DJ, Kowdley KV. Cardiopulmonary consequences of transjugular intrahepatic portosystemic shunts: role of increased pulmonary artery pressure. J Clin Gastroenterol. 2004;38:590-594. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 166. | Boyer TD, Haskal ZJ. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41:386-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 167. | Hadengue A, Benhayoun MK, Lebrec D, Benhamou JP. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528. [PubMed] [Cited in This Article: ] |
| 168. | Katsuta Y, Zhang XJ, Kato Y, Shimizu S, Komeichi K, Ohsuga M, Higashi H, Satomura K, Takano T. Hemodynamic features and impaired arterial oxygenation in patients with portopulmonary hypertension. Hepatol Res. 2005;32:79-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 169. | Kuntzen C, Gülberg V, Gerbes AL. Use of a mixed endothelin receptor antagonist in portopulmonary hypertension: a safe and effective therapy. Gastroenterology. 2005;128:164-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 170. | Hoeper MM, Halank M, Marx C, Hoeffken G, Seyfarth HJ, Schauer J, Niedermeyer J, Winkler J. Bosentan therapy for portopulmonary hypertension. Eur Respir J. 2005;25:502-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 140] [Article Influence: 7.4] [Reference Citation Analysis (0)] |