Published online Dec 14, 2013. doi: 10.3748/wjg.v19.i46.8789
Revised: September 14, 2013
Accepted: September 16, 2013
Published online: December 14, 2013
Amoxicillin/clavulanate is a synthetic penicillin that is currently commonly used, especially for the treatment of respiratory and cutaneous infections. In general, it is a well-tolerated oral antibiotic. However, amoxicillin/clavulanate can cause adverse effects, mainly cutaneous, gastrointestinal, hepatic and hematologic, in some cases. Presented here is a case report of a 63-year-old male patient who developed cholestatic hepatitis after recent use of amoxicillin/clavulanate. After 6 wk of prolonged use of the drug, he began to show signs of cholestatic icterus and developed severe hyperbilirubinemia (total bilirubin > 300 mg/L). Diagnostic investigation was conducted by ultrasonography of the upper abdomen, serum tests for infection history, laboratory screening of autoimmune diseases, nuclear magnetic resonance (NMR) of the abdomen with bile duct-NMR and transcutaneous liver biopsy guided by ultrasound. The duration of disease was approximately 4 mo, with complete resolution of symptoms and laboratory changes at the end of that time period. Specific treatment was not instituted, only a combination of anti-emetic (metoclopramide) and cholestyramine for pruritus.
Core tip: This report describes a case of acute cholestatic hepatitis caused by the use of amoxicillin/clavulanate. This case presented an unusual evolution, characterized by severe hyperbilirubinemia and cholestatic symptoms without the development of hepatic failure, and with total resolution requiring no specific treatment. There are few case reports in the literature that describe a similar clinical condition due to drug-induced cholestatic hepatitis.
- Citation: Beraldo DO, Melo JF, Bonfim AV, Teixeira AA, Teixeira RA, Duarte AL. Acute cholestatic hepatitis caused by amoxicillin/clavulanate. World J Gastroenterol 2013; 19(46): 8789-8792
- URL: https://www.wjgnet.com/1007-9327/full/v19/i46/8789.htm
- DOI: https://dx.doi.org/10.3748/wjg.v19.i46.8789
Amoxicillin/clavulanate is a synthetic penicillin that is currently commonly used, especially for the treatment of respiratory and cutaneous infections. The addition of clavulanate to amoxicillin provides action against bacteria that produce beta-lactamase, conferring a wide spectrum against gram-positive and -negative bacteria for the drug[1]. However, this combination considerably changes the frequency of collateral effects, as described in a study by Francesco Salvo et al[1] that examined the frequency of drug reactions in six Italian regions from January 1988 to June 2005. Their study showed that the percentage of gastrointestinal, hepatic and hematological reactions was significantly higher for amoxicillin/clavulanic acid (13%, 4% and 2%, respectively) than for amoxicillin (7%, 1% and 1%, respectively)[1].
With respect to hepatic side effects, cases of drug-induced hepatitis by amoxicillin/clavulanate have been reported since the 1980s, typically with a benign course. Approximately 23% of individuals on amoxicillin/clavulanate experience non-significant increases in hepatic enzymes[2]. However, a small number of severe episodes have been described, some of which are characterized by fulminant hepatitis, a disease that leads to death or the need for liver transplant[3].
Presented here is a case report of a 63-year-old male patient who developed cholestatic hepatitis after use of amoxicillin/clavulanate.
The male, a 63-year-old patient was admitted to the Hospital Renascentista on September 1, 2012, with a history of jaundice, choluria, fecal acholia, generalized pruritus, malaise, hyporexia and sporadic nausea without associated vomiting for five days.
The patient had hypertension for 10 years, dyslipidemia for 3 years, a recent diagnosis of altered fasting blood sugar, oligosymptomatic benign prostatic hyperplasia for 3 years and was overweight. He took 50/12.5 mg of atenolol/chlorthalidone once a day. He indicated that it had been approximately 45 d since he had used a topical corticoid for 15 d for acute otitis, and denied using any other medications. He had no history of trauma or surgery, and no epidemiological history of note. He was a non-smoker and drank alcohol on the weekend (three cans of beer on Saturdays and Sundays), but did not drink in the periods preceding the beginning of the symptoms.
Upon physical examination at admission, the patient was jaundiced (2+/4) with small traumatic lesions on the skin, was afebrile, normotensive (AP: 130/80 mmHg) with a heart rate of 80 beats/min, eupneic without changes in pulmonary auscultation and showed normal findings on abdominal examination with no visceromegaly.
The admission tests indicated Hb: 130.6 g/L, leukocytes: 7200 cells/mm3 (normal differential), platelets: 248000 cells/mm3, fasting glycemia: 1100 mg/L, creatinine: 9 mg/L, urea: 290 mg/L, Na: 137 mEq/L, K: 3.8 mEq/L, Mg: 18 mg/L, Ca: 42 mg/L, blood gases: normal, CRP: 66 mg/L, amylase: 40 IU/L, lipase: 35I U/L, AST: 78 IU/L, ALT: 200 IU/L, alkaline phosphatase: 60 IU/L, GGT: 33 IU/L, albumin: 33 g/L, complete coagulation profile: normal, LDH: 2 94I U/L, total bilirubin: 83 mg/L, direct bilirubin: 50.1 mg/L, reticulocytes: 0.9%, haptoglobin: 1440 mg/L (160-2200 mg/L).
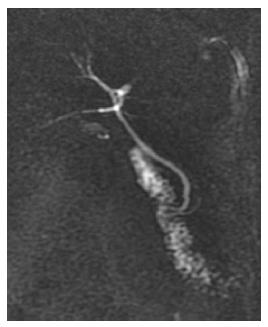
Diagnostic investigation began with ultrasonography of the upper abdomen, which demonstrated only cholesterolosis of the biliary vesicles. Serological tests for hepatitis A, hepatitis B, hepatitis C, hepatitis E, cytomegalovirus, Epstein-Barr, dengue, leptospirosis and HIV were all negative. Auto-immune analysis demonstrated negative anti-nuclear factor, anti-smooth muscle and anti-mitochondria, and normal serum IgG and serum IgM. Nuclear magnetic resonance (NMR) of the abdomen with bile duct-NMR was subsequently requested, which demonstrated constricted biliary vesicles (Figure 1).
The Council for International Organizations of Medical Sciences (CIOMS) score was +9 points and the Clinical Diagnostic Scale (CDS) score was +11 points.
During hospitalization, the patient developed a progressive worsening of hyperbilirubinemia (Table 1), pruritus, malaise and nausea, where he received symptomatic treatment with cholestyramine 4 g four times per day and metoclopramide when necessary.
| 9/1 | 9/3 | 9/6 | 9/9 | 9/10 | 9/11 | 9/18 | 9/22 | 9/26 | |
| AST (IU/L) | 78 | 89 | 80 | 75 | 51 | 50 | 63 | 77 | 70 |
| ALT (IU/L) | 200 | 245 | 160 | 130 | 69 | 59 | 65 | 89 | 82 |
| BT/BD (mg/L) | 80.3/50.1 | 100.9/70.0 | 110.3/70.9 | 150.6/90.0 | 180.3/110.0 | 210.9/140.0 | 250.9/160.0 | 280.9/180.0 | 310.2/200.0 |
| AP (IU/L) | 60 | 80 | 110 | 140 | 193 | 188 | 239 | 300 | 311 |
| GGT (IU/L) | 33 | 65 | 90 | 123 | 150 | 134 | 175 | 223 | 226 |
| INR | 1.01 | 1.00 | 1.12 | 1.1 | 1.0 | 1.2 | 1.0 | 1.0 | 1.0 |
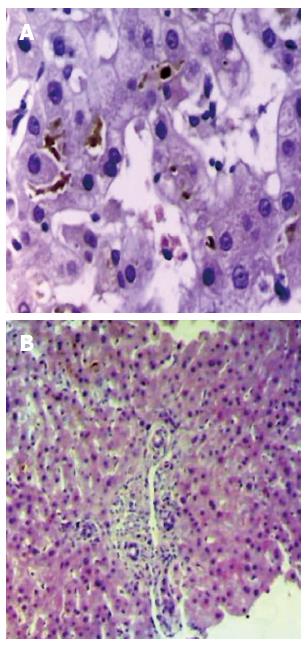
Because there was no clinical improvement on day 30, the patient was more extensively questioned. Additionally, the accompanying family members were asked to bring all recent medical documents. A prescription was found for amoxicillin-clavulanate 500 mg 3 times a day for twenty-one days, which had been initially used forty-five days ago, together with a topical corticoid to treat acute otitis. The presence of these drugs led to a suspected diagnosis of drug-induced cholestatic hepatitis, which was confirmed by transcutaneous liver biopsy (Figure 2).
The patient began to show improvement, both clinically and based on laboratory results, after thirty days of hospitalization and was discharged with the use of cholestyramine 4 g four times per day, and for recommendation of outpatient follow-up.
Four months after the onset of symptoms, he became asymptomatic with jaundice and other previous changes resolved, normal routine liver tests, and he began receiving only previous chronic anti-hypertensive therapy.
Amoxicillin/clavulanate is a widely used antibiotic that is associated with adverse effects, especially of the cutaneous, gastrointestinal, hepatic and hematologic types[1]. The incidence of hepatic damage by amoxicillin/clavulanate is greater than that associated with amoxicillin administration alone (1.7 vs 0.3 for every 10000 prescriptions)[1-4]; predominantly cholestatic lesions, although isolated mixed and hepatocellular lesions also occur[2,5-7]. There are also reports in the literature of patterns of granulomatous lesion secondary to the use of the medication in question[8].
Histopathological examination usually reveals centrilobular or panlobular cholestasis and inflammation, predominantly lymphocytic, portal and periportal, with neutrophils and eosinophils frequently present[2,3,6]. Other biopsy findings include degeneration and necrosis of ductal epithelial cells, ductopenia[4] and vacuolization and necrosis of hepatocytes[5,6], all in addition to granulomatous inflammatory process[8].
The pathogenic events that cause lesions due to the use amoxicillin/clavulanate require further study[3], but it is believed that idiosyncratic immunoallergic mechanisms are the underlying causes[3,6,7,9-11]. The common presence of eosinophils in the inflammatory infiltrate[3], the coexistence of manifestations of hypersensitivity, such as skin rash and hypereosinophilia[3], the documentation of the involvement of specific autoantibodies (anti-mitochondrial type 6, anti-LKM2 and anti-LM antibodies)[3] and class II HLA antigens (DRB1*1501-DRB5*0101-DQB1*0602)[11] reinforce the hypothesis that immune aggression is involved in the lesions formed due to amoxicillin-clavulanate use.
The risk factors for hepatotoxicity caused by amoxicillin/clavulanate include male sex, associated alcohol consumption, repeated courses of the drug, concomitant consumption of other hepatotoxic substances[2] and age over 55 years[7]. Treatment duration has been included as a predisposing factor in some reviews[3].
The clinical characteristics are predominantly cholestatic signs and symptoms, which include malaise, hyporexia, nausea, vomiting, jaundice, choluria, fecal acholia, cutaneous pruritus and, less commonly, painful hepatomegaly. Manifestations associated with hypersensitivity can occur, such as skin rash and fever, with an incidence as high as 50%[12,13]. The symptoms can begin in any period after the end of treatment, but typically appear between 4 and 10 wk and are self-healing, as they are resolved in 4-16 wk. Reports of chronification, as described by Ryley et al[14], are extremely rare.
Severe hyperbilirubinemia, changes in laboratory liver function blood tests and neurological alterations constitute the criteria for a poor prognosis, with the possibility of the development of fulminant hepatitis[3,6,9].
Treatment consists mainly of support and should attend to various aspects of hepatic lesions. It is common for patients to become dehydrated due to decreased fluid intake and vomiting. Therefore, the evaluation of the volemic status is essential and should be corrected rapidly if necessary. Additionally, the cholestatic symptoms can become limiting and require prescriptions for symptomatic patients, such as anti-emetics and analgesics, in addition to medications to control pruritus. Generally, cholestyramine, anti-histamines, ursodeoxycholic acid and sertraline are used dependent on the intensity of symptoms and the experience of the service with the use of the drugs.
Due to the likely immunological mechanism of hepatic lesions, including hypersensitivity reactions mediated by eosinophils, some authors advocate the use of a systemic corticoid in the treatment of severe cases and in those with potential severity, such as hyperbilirubinemic individuals[6]. However, there is no evidence of reduced morbidity.
This study reported the case of a 63-year-old patient who began to show signs of cholestatic icterus after 6 wk of prolonged use of amoxicillin/clavulanate. The patient developed severe hyperbilirubinemia, but did not meet other criteria of severity. The time of disease was approximately four months, with complete resolution of symptoms and laboratory changes. The patient did not receive any specific treatment, only a combination of anti-emetics (metoclopramide) and cholestyramine for pruritus.
Of the risk factors for hepatotoxicity due to the drugs, advanced age, male sex, alcohol drinking and prolonged antibiotic therapy were all present in this case. The period of the onset of symptoms, the clinical characteristics and the time for complete recovery were in accordance with other reported cases. The degree of hyperbilirubinemia is important in the laboratory profile, with few described cases of total bilirubin reaching values higher than 300 mg/L[6]. Although there is a possible relation with severity, our patient did not show any signs of hepatic dysfunction. A complete history was taken, as described, to rule out other causes of hepatotoxicity. Based the Council for International Organizations of Medical Sciences score (CIOMS) (+9 points - very likely association)[15], the Clinical Diagnostic Scale (CDS) score (+11 points - possible association)[16] and information from liver biopsy, this case had a high probability of hepatotoxicity due to drug use.
The biopsy findings, besides the cholestasis, were typical of hepatitis due to amoxicillin-clavulanate, periportal lymphocytic inflammation with damage to hepatocytes, alterations found in the pathology[2-6].
A relevant factor in the case, which made the diagnosis difficult, was the denial of the patient to taking the medication. Thus, this report demonstrates the importance of thorough anamnesis and careful verification for antibiotics and/or other drugs use.
P- Reviewers: Jirsa M, Menezes GB, Ruiz-Gaspa S S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN
| 1. | Salvo F, Polimeni G, Moretti U, Conforti A, Leone R, Leoni O, Motola D, Dusi G, Caputi AP. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemother. 2007;60:121-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Dandakis D, Petrogiannopoulos C, Hartzoulakis G, Flevaris C. Lagoutari D, Drakogiorgos D, Zaharof A, Barbati K. Cholestatic hepatitis associated with amoxicillin-clavulanic acid combination. A case report. Ann Gastroentol Hepatol. 2002;15 Suppl 1:85-87. [Cited in This Article: ] |
| 3. | Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785-1790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Kim JS, Jang YR, Lee JW, Kim JY, Jung YK, Chung DH, Kwon OS, Kim YS, Choi DJ, Kim JH. A case of amoxicillin-induced hepatocellular liver injury with bile-duct damage. Korean J Hepatol. 2011;17:229-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Farrokhseresht R. Liver Associated Injury of Amoxicillin-Clavulanic Acid. SEMJ. 2001;2:53-56. [Cited in This Article: ] |
| 6. | Herrero-Herrero JI, García-Aparicio J. Corticosteroid therapy in a case of severe cholestasic hepatitis associated with amoxicillin-clavulanate. J Med Toxicol. 2010;6:420-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Zaidi SA. Hepatitis associated with amoxicillin/clavulanic acid and/or ciprofloxacin. Am J Med Sci. 2003;325:31-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Silvain C, Fort E, Levillain P, Labat-Labourdette J, Beauchant M. Granulomatous hepatitis due to combination of amoxicillin and clavulanic acid. Dig Dis Sci. 1992;37:150-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Cundiff J, Joe S. Amoxicillin-clavulanic acid-induced hepatitis. Am J Otolaryngol. 2007;28:28-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Schey R, Avni Y, Bruck R, Shirin H. History of drug-induced hepatitis and risk of amoxicillin/clavulanate-induced hepatotoxicity. Ann Pharmacother. 2001;35:1142-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, Brenard R, Sempoux C, Michielsen PP, Yap PS. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117:1181-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Brown SJ, Desmond PV. Hepatotoxicity of antimicrobial agents. Semin Liver Dis. 2002;22:157-167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Hautekeete ML, Brenard R, Horsmans Y, Henrion J, Verbist L, Derue G, Druez P, Omar M, Kockx M, Hubens H. Liver injury related to amoxycillin-clavulanic acid: interlobular bile-duct lesions and extrahepatic manifestations. J Hepatol. 1995;22:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Ryley NG, Fleming KA, Chapman RW. Focal destructive cholangiopathy associated with amoxycillin/clavulanic acid (Augmentin). J Hepatol. 1995;23:278-282. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272-276. [PubMed] [Cited in This Article: ] |
| 16. | Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26:664-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |