Article Text
Abstract
Objective To assess the effect of sildenafil, a phosphodiesterase type 5 inhibitor, on digital ulcer (DU) healing in systemic sclerosis (SSc).
Methods Randomised, placebo-controlled study in patients with SSc to assess the effect of sildenafil 20 mg or placebo, three times daily for 12 weeks, on ischaemic DU healing. The primary end point was the time to healing for each DU. Time to healing was compared between groups using Cox models for clustered data (two-sided tests, p=0.05).
Results Intention-to-treat analysis involved 83 patients with a total of 192 DUs (89 in the sildenafil group and 103 in the placebo group). The HR for DU healing was 1.33 (0.88 to 2.00) (p=0.18) and 1.27 (0.85 to 1.89) (p=0.25) when adjusted for the number of DUs at entry, in favour of sildenafil. In the per protocol population, the HRs were 1.49 (0.98 to 2.28) (p=0.06) and 1.43 (0.93 to 2.19) p=0.10. The mean number of DUs per patient was lower in the sildenafil group compared with the placebo group at week (W) 8 (1.23±1.61 vs 1.79±2.40 p=0.04) and W12 (0.86±1.62 vs 1.51±2.68, p=0.01) resulting from a greater healing rate (p=0.01 at W8 and p=0.03 at W12).
Conclusions The primary end point was not reached in intention-to-treat, partly because of an unexpectedly high healing rate in the placebo group. We found a significant decrease in the number of DUs in favour of sildenafil compared with placebo at W8 and W12, confirming a sildenafil benefit.
Trial registration number NCT01295736.
- Autoimmune Diseases
- Patient perspective
- Treatment
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Statistics from Altmetric.com
Introduction
Ischaemic digital ulcers (DUs) are an expression of the severity of the microangiopathy in patients with systemic sclerosis (SSc). DUs are a frequent complication affecting about 50% of patients with SSc.1 ,2 In cross-sectional studies involving patients with SSc, the frequency of ischaemic DUs was 12–16%,3 ,4 with a major impact on hand function and quality of life.5
Effective therapy for DUs remains elusive. Intravenous iloprost has demonstrated a positive effect on DU healing.6 Recently, bosentan, an oral endothelin receptor antagonist, tested in two large randomised controlled studies, showed a benefit on the occurrence of new DUs, but did not show any effect on time to complete or partial healing of DUs in SSc.7 ,8
Sildenafil, a phosphodiesterase type 5 (PDE-5) inhibitor, acts by inhibiting the breakdown of cyclic guanosine monophosphate. Elevated cyclic guanosine monophosphate reduces levels of intracellular calcium, thereby causing relaxation of smooth muscle cells. It was reported that sildenafil led to a 400% increase of the flow velocity in digital capillaries in patients with severe Raynaud's phenomenon (RP).9 Because of their vasodilating, angiogenic and decreased platelet aggregation properties, PDE-5 inhibitors may have a potentially beneficial effect on DU healing.10 In two uncontrolled pilot studies sildenafil had a promising effect on DU healing.11 ,12 In a small double-blind randomised cross-over trial focusing on RP, which included patients with connective tissue diseases, mostly SSc, all the ischaemic lesions present healed during treatment with tadalafil.13 These findings suggested an effect of sildenafil on DU healing in patients with SSc, which needed to be validated in a larger controlled study.
Methods
Study design
This was a prospective, longitudinal, randomised, comparative, double-blind, two-parallel-arm, placebo-controlled study conducted in 25 centres in France. The aim of the study was to assess the effect of sildenafil on time to healing and healing rate in patients with SSc with active DUs. Patients meeting the inclusion criteria were randomly allocated in two groups (ratio 1:1) and received either sildenafil 20 mg three times a day or matching placebo for 12 weeks. Randomisation was stratified by centre and by the number of DUs at entry (<three DUs, ≥three DUs) using a centralised website. This ensured that patients with a more severe DU disease were well balanced between the two groups. Patients were asked to visit the physician every 4 weeks. The main assessment criterion was time to healing of each DU.
Study oversight
The study started in February 2011 and was completed in August 2013.
Patients
All patients with SSc, diagnosed according to the American College of Rheumatology criteria,14 or the LeRoy and Medsger criteria for diffuse or limited subsets classification,15 and presenting with at least one ischaemic DU on their fingers distal to the proximal interphalangeal joint, could enter the study. A DU was defined as present (active) in the case of a break in the skin with a loss of epithelialisation on the distal finger surface of ischaemic origin according to the physician and not located over subcutaneous calcifications or over extensor surfaces of joints. In the case of an underlying scab, the presence of a DU was considered based on the clinical judgement of the physician. All the inclusion and exclusion criteria can be found on the clinical.trial.gov web site (http://clinicaltrials.gov/show/NCT01295736). Patients receiving bosentan at enrolment could enter the study provided the dose of bosentan had been stable for at least 1 month before inclusion. Patients had to withdraw from the study at any time in the event of DU worsening.
Evaluation of ischaemic DUs
At each patient visit (week (W) 0, W4, W8 and W12), the physician had to collect data on each ulcer separately. This included location (hand, finger and finger surface involved), size and local complications such as gangrene, osteomyelitis or skin infection. The largest diameter was measured and expressed in millimetres. Healing was defined as complete re-epithelialisation. The evaluation of the duration of the DUs present at baseline was based on the patient's estimation.
Other evaluations
History of SSc was collected at entry. Factors that could worsen digital ischaemia, such as smoking, use of vasoconstrictive drugs, or occupational exposure to cold were reported. Date of entry in the study was taken into account to look for any seasonal effect on healing. Hand disability was assessed by the Cochin hand function score.16 Global disability was assessed by the Health Assessment Questionnaire Disability Index (SSc HAQ-DI). Patients were asked to evaluate the severity of RP according to frequency, pain, and disturbance within the previous 4 weeks on a 100-mm visual analogue scale (VAS; 0=not severe and 100=very severe). Patient compliance with treatment was only assessed by counting unused medication at each visit.
Statistical analysis
Results are expressed as mean±SD (m±SD) and numbers (percentage). The primary end point was the time to healing, which was determined for each DU present at entry (W0) as the delay between W0 and the first visit on which healing was observed (re-epithelisation of the DU assessed by the investigator). A total of 144 DUs (72 in each group) with a total number of events (DU healing) of 81 would ensure an 80% power to detect a difference between proportions of events P1 (placebo) of 0.30 and P2 (sildenafil) of 0.50 with a constant HR of 1.737; this assumed no dropouts before time t. Tests were two-sided with α risk set at 0.05. Assuming at least 50% of patients would be suffering from ≥two DUs at W0, an intracluster correlation of 0.30, and a dropout rate of 10%, 120 patients needed to be included in the study.
The primary analysis was conducted on an intention-to-treat (ITT) basis. A per protocol (PP) analysis was performed in patients with satisfactory compliance (≥66% for each time interval between visits). A subset of DUs ≥2 mm and evolving for 1–3 months at entry in the study was also examined on an ITT basis.
Since evolution of DUs could potentially be correlated at individual patient level, we performed a survival analysis of clustered data using marginal proportional hazards models17 and sandwich-type estimators (an observation being defined as a DU and a cluster as a patient)18 ,19 adjusted for DU disease severity, assessed by the number of DUs present at W0. The seasonal effect was tested in two separate models (cold season: 1 September to 30 March; warm season: 1 April to 31 August). Sildenafil and placebo were also compared in two separate groups: patients with or without treatment by bosentan.
The number of DUs per patient at each visit and the change in the number of DUs between W0 and W12 were compared using Poisson regression models. Healing rates of DUs present at W0 and the proportion of patients whose DUs present at W0 were completely healed at W12 were compared between groups using a logistic regression model for clustered data. Changes in pain, disability, hand function and RP severity between W0 and W12 were analysed using mixed models for repeated measures. Two-sided p values <0.05 are considered to indicate statistical significance, and 95% CIs are reported where relevant.
Statistical analyses were performed with SAS software V.9.3 (SAS Institute, Cary, North Carolina, USA).
Results
Patients
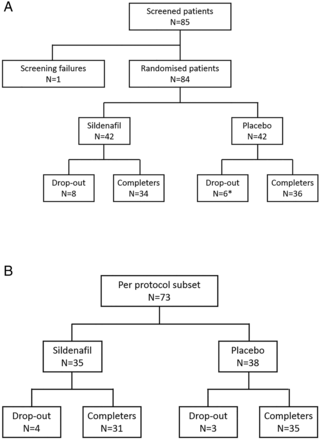
Eighty-four patients entered the study and were randomised to receive either sildenafil 20 mg three times a day (N=42) or placebo (N=42) (figure 1). Fourteen patients withdrew from the study, mainly due to adverse events. One patient withdrew prematurely without evaluation of DU after entry. Thus, the ITT analysis involved 83 patients (N=42 for sildenafil and N=41 for placebo). Three patients from the placebo group and seven from the sildenafil group had poor compliance with the study drug. Thus, the PP analysis involved 73 patients (N=35 for sildenafil and N=38 for placebo).
Trial profile. (A) Intention-to-treat population. (B) Per protocol subset. *One premature drop out patient in the placebo group had no evaluation of discontinuation beyond inclusion.
The characteristics of patients at baseline are described in the ITT population (table 1).
Patients’ characteristics at entry in the study—intention-to-treat population
At entry in the study (W0), the patients presented a total of 192 DUs (89 in the sildenafil group and 103 in the placebo group) evolving for 161±401 days (median 56 days). The mean number of DUs per patient was 2.1±1.4 in the sildenafil group and 2.5±2.1 in the placebo group. The mean largest diameter of DUs was 4.7±3.2 mm and 98.8% of patients had at least one DU with a largest diameter ≥2 mm. Pain intensity was rated 58.8±26.8 on a 100-mm VAS during the week preceding entry in the study and 47.1±27.8 on the day of the visit. SSc-HAQ-DI score was 1.0±0.8. Hand disability was rated 29.8±19.1 on the Cochin hand function score (table 2).
Secondary end points—intention-to-treat analysis
Primary end point
The HR for DU healing was 1.33 (CI 0.88 to 2.00) (p=0.18) in an unadjusted model and 1.27 (CI 0.85 to 1.89) (p=0.25) in a model adjusted for the number of DUs at entry, in favour of sildenafil. Similar results were found in the subset of DUs ≥2 mm and evolving for 1–3 months at entry in the study; HR 1.21 (CI 0.61 to 2.41) and 1.13 (CI 0.52 to 2.46), for non-adjusted and adjusted models.
Secondary end points
The mean number of DUs present per patient regularly decreased over time in both groups but differences between groups were significantly in favour of sildenafil at W8 (ratio 0.69 (CI 0.47 to 0.99), p=0.04) and W12 (ratio 0.57 (CI 0.37 to 0.88), p=0.01) (table 3). The healing rate for DUs present at baseline was greater in the sildenafil group at W8 (OR 1.82 (CI 1.15 to 2.88), p=0.01) and W12 (OR 1.78 (CI 1.06 to 2.97), p=0.03) (figure 2). At W12, all the DUs present at baseline had healed in 29 patients (70.3%) of the sildenafil group and 23 patients (60.5%) of the placebo group (OR 1.50 (CI 0.52 to 4.37), p=0.45) (figure 3). New DUs occurred between W4 and W12 in eight patients (21.6%) in the sildenafil group and in 15 patients (39.5%) in the placebo group (OR 0.42 (CI 0.15 to 1.17), p=0.10). Twenty-five patients (67.6%) in the sildenafil group and 18 patients (48.7%) in the placebo group had no DUs at W12 (OR 2.20 (CI 0.86 to 5.65), p=0.10), meaning that all DUs present at baseline had healed and either no new DUs had occurred or any new DUs had healed during the study period. DU complications were reported in four patients (one patient with cutaneous infection and gangrene in the sildenafil group, three patients with cutaneous infection in the placebo group). Pain, hand disability and the severity of RP decreased over time without difference between groups (table 2). HAQ-DI did not vary over the study period.
Secondary end points—intention-to-treat analysis
Healing rate of digital (DUs) present at entry in the study (intention-to-treat analysis). Hatched columns, placebo group; Solid black columns, sildenafil group.
Example of digital ulcer evolution during the clinical trial in a patient who was assigned to the sildenafil group. (A) at randomisation; (B) at week 4; (C) at week 8; and (D) at week 12.
Per protocol analysis
The HR for DU healing was 1.49 (0.98 to 2.28) (p=0.06) in an unadjusted model and 1.43 (0.93 to 2.19) (p=0.10) in a model adjusted for the number of DUs at entry. The mean number of DUs per patient decreased over time and was lower in the sildenafil group than in the placebo group at W8 (OR 0.64 (CI 0.43 to 0.94), p=0.03) and W12 (OR 0.47 (CI 0.29 to 0.76), p=0.002). The proportion of healed DUs was 35% at W4, 69% at W8 and 82% at W12 in the sildenafil group and 29%, 51% and 64%, respectively, in the placebo group. The difference between groups was statistically significant at W8 (OR 2.18 (CI 1.31 to 3.62), p=0.003) and W12 (OR 2.62 (CI 1.50 to 4.56), p=0.0007). Thirty-two patients in the sildenafil group and 36 in the placebo group remained in the study until W12. All DUs that were present at entry were healed in 24/32 patients (75.0%) of the sildenafil group and in 21/36 patients (58.3%) of the placebo group (OR 2.24 (CI 0.69 to 7.25), p=0.18); 23/32 patients (71.9%) in the sildenafil group and 16/36 patients (45.7%) in the placebo group had no DUs at W12 (OR 3.06 (CI 1.03 to 9.09), p=0.03). New DUs occurred between W4 and W12 in 6/32 patients (18.8%) in the sildenafil group and in 14/36 patients (38.9%) in the placebo group (OR 0.36 (CI 0.12 to 1.10), p=0.07).
Seasonal effect
We found no effect of the season of enrolment on the healing rate (p=0.34) and no difference between groups in seasonal subsets (p=0.09 during the warm season, p=0.38 during the cold season).
Subgroup of patients receiving bosentan concomitantly
In the ITT population, a subgroup of 28 patients with 80 DUs was receiving bosentan concomitantly with the study drug. In this subgroup time to healing was shorter in the sildenafil group than in the placebo group (HR 1.75 (95% CI 0.94 to 3.26), p=0.08) in an unadjusted model. The difference was not significant in a model adjusted for the severity of the ulcerative disease (p=0.41).
Safety
Adverse events led to study discontinuation for five patients in the sildenafil group (drowsiness, syncope, headache, facial oedema, rash: n =1 each) and three in the placebo group (leg oedema, headache and vomiting, dizziness: n=1 each).
Discussion
This is the first, well-designed, randomised, placebo-controlled, parallel-group study conducted to evaluate the efficacy of sildenafil on healing of ischaemic DUs in SSc. Each DU was considered as an individual entity and was separately evaluated. The primary end point evaluating the time to DU healing was not reached. Nevertheless, the number of DUs was significantly reduced in the sildenafil group at W8 and W12 when compared with the placebo group, reflecting a higher healing rate in the sildenafil group at W8 and W12.
Why sildenafil could be useful in DU healing
Four randomised controlled studies evaluating the effect of PDE-5 inhibitors on RP have previously been published,9 ,20 ,21 ,13 three of which showed a benefit. Shenoy et al13 evaluated tadalafil 20 mg once a day versus placebo in a double-blind randomised cross-over trial over a 6-week period of treatment in 23 patients with SSc and found a significant improvement in DU healing. In the tadalafil group, 20/20 DUs healed, whereas 3/13 healed in the placebo group. In an uncontrolled pilot study conducted by Brueckner et al,11 ischaemic lesions began to exhibit signs of healing during treatment with PDE-5 inhibitors. Della Rossa et al12 also reported promising results of an uncontrolled study of the efficacy of sildenafil in real life conditions in 15 patients with SSc.
The potential reasons for not reaching the primary end point
The primary end point evaluating the time to DU healing was not reached. There are several possible explanations:
An unexpectedly high healing rate in the placebo group: The high healing rate observed in the placebo group was unexpected. Our hypothesis was derived from the Korn et al7 study (designed to compare bosentan and placebo) showing about 30% of DUs healed after 3 months in the placebo group. Surprisingly, in our study, 66% of DUs present at baseline were healed at W12 in the placebo group. Since all participating centres were highly experienced in the treatment of SSc and DUs, the local DU treatment provided was certainly more effective than the treatment given 10 years ago when the Korn et al7 study was conducted.
Inaccurate evaluation of time to healing: The investigators diagnosed each DU as healed or not healed at each visit (at W4, W8 and W12). This means that a DU that was healed at W8+5 days and a DU that was healed at W8+25 days would both have been considered as not healed at W8 and healed at W12. Such a difference could not be detected by the investigators.
Effect of calcium channel blockers?: Calcium channel blockers are recommended for the treatment of RP, which is a very common feature in SSc. In a 16-week, randomised, double-blind, placebo-controlled study comparing intravenous infusions of iloprost on three consecutive days with a further single infusion at W8 to oral nifedipine for 16 weeks, a similar effect was found in both groups in terms of reduction in the mean number of digital lesions (a mixture of DUs, fissures and paronychia) at W16.22 Moreover, it has also been suggested in a retrospective study,2 that the use of vasodilators (particularly calcium channel blockers) may reduce the risk of a first DU episode in patients with SSc. In our study, 45.1% of the patients in the sildenafil group and 54.9% of the patients in the placebo group were receiving calcium channel blockers. This may also have contributed to the high rate of DU healing observed in the placebo group.
Relevance of secondary end points
The number of DUs was lower in the sildenafil group at W8 and W12 when compared with the placebo group, reflecting a higher healing rate in the sildenafil group at W8 and W12, a lower, albeit non-significant incidence of new DUs and/or a quicker healing of newly occurring DUs during the study period. These results strongly suggest that sildenafil improved the ulcerative disease and could be of interest in patients with SSc suffering from DUs, most likely by increasing the flow velocity of digital capillaries.9
Interest of a sildenafil and bosentan combination
Analysing in the ITT population a subgroup of patients who were on bosentan at the time of the randomisation, we found that time to healing was significantly shorter in the sildenafil+bosentan subgroup than in the placebo+bosentan subgroup. This suggests that the combination of sildenafil and bosentan might have a beneficial effect on the healing of DUs. More evidence and further studies will of course be required to confirm this finding, which is consistent with what is known in pulmonary arterial hypertension.
Other outcomes
Pain and hand disability decreased in both groups without differences between groups. In the placebo group this decrease could be due to good local care. RP improved in both groups without any statistical difference. The study was not designed to compare the effect of sildenafil and placebo on RP but this finding also reflects the high placebo effect on RP. In addition, RP was evaluated using a VAS but not using a precise evaluation tool such as the Raynaud's Condition Score.
The reason we did not find any seasonal effect on the healing process is probably due to the good educational level of patients seen in tertiary clinics, who are likely to have developed highly protective habits against the cold and humidity in winter and autumn, which may help DU healing.
Strengths and limitations
The main strengths of our study are the number of DUs, the stratified randomisation based on the number of DUs at entry, assumed to reflect the severity of the ulcerative disease, and the estimation of the HR between groups using a proportional risks Cox model for clustered data. This type of analysis is known to be more conservative than Cox models that do not take into account the clustering of data. Healing was defined as complete re-epithelialisation of the DU. Time to healing is undoubtedly the most clinically relevant criterion but it appeared to be difficult to evaluate in ambulatory patients and further studies might prefer to choose the number of DU at each time point as the primary end point.
Among other potential limitations is possibly the relatively low cut-off value for good compliance that we used (≥66% for each time interval between visits) and the use of too low a dosage of sildenafil. Using sildenafil in pulmonary arterial hypertension, it is possible to increase the dose up to 80 mg three times daily with a haemodynamic benefit.23 It would perhaps be beneficial to increase the dose of sildenafil after 4 weeks of treatment if no effect or an insufficient effect were observed. Nevertheless, this would require further studies. A second limitation is that the expected number of patients was not reached even after prolongation of the recruitment period, which could not be increased due the use of all the funding. Nevertheless, DUs were individually considered in this study and the expected number of DUs was reached (expected N=144, observed N=192). The sample size was, however, calculated for a planned one-sided test on the primary end point and two-sided tests were actually performed. This as well as the unexpectedly high rate of DU healing in the placebo group makes the study underpowered.
Conclusion
This placebo-controlled randomised study did not demonstrate that sildenafil 20 mg three times daily shortened the time to healing of DUs in SSc. Nevertheless, the results were in favour of sildenafil in the ITT and PP populations with a significantly lower number of DUs at W8 and W12, confirming a sildenafil benefit. The results are promising in the subgroup of patients receiving bosentan concomitantly.
Acknowledgments
The authors thank the Groupe Francophone de Recherche sur la Sclérodermie (GFRS) and the French Scleroderma Patients’ Association (Association des Sclérodermiques de France ((ASF)) which helped to distribute the study protocol to its members. The authors also thank Nicholas Barton for his advice on editing the manuscript.
References
Footnotes
Handling editor Tore K Kvien
Collaborators SEDUCE study group: Thomas Quemeneur, Valenciennes; Marie-Hélène Balquet, Lens; Géraldine Wojtasik, Marc Lambert, David Launay, Hélène Maillard, Sandrine Morell-Dubois, Noémie Le Gouellec, Lille; Isabelle Marie, Rouen; Nathalie Tieulie, Nice; Christophe Deligny, Fort de France; Boris Bienvenu, Caen; Dominique Farge-Bancel, Alice Berezne, Isabelle Lazareth, Sondess Hadj Khelifa, Kiet Tiev, Jean Cabane, Baptiste Hervier, Zahir Amoura, Yannick Allanore, Paris; Brigitte Granel, Marseille; Jean-Philippe Arnault, Amiens; Sabine Berthier, Dijon; Claire Cazalets, Rennes; Arsène Mekinian, Bondy; Kim Ly, Agnès Sparsa, Limoges; Jean Sibilia, Strasbourg; Jacques Ninet, Lyon, France.
Contributors EH participated in study conception, design and supervision; data acquisition, analysis and interpretation; and drafting, revision and approval of the report. PiCl participated in study conception and design; data acquisition, analysis and interpretation; drafting and approval of the report; and did all the statistical analyses. P-YH, PaCa, CA, EC, PJ, LM, VQ, A-LF, UM-P, RJ, AM, BG, ED, DF-B, AM, JA and HD-C participated in data acquisition, analysis and interpretation; revision and approval of the report. The first draft of the manuscript was written by the first and last authors, and was edited and revised by all the other authors.
Funding The sponsor was the University Hospital of Lille (Fédération de Recherche Clinique). This study was supported by an unrestricted research grant from PFIZER.
Competing interests
EH received fees from Pfizer, Actelion, GlaxoSmithKline <€10 000 each; PaCa received fees from Actelion, <€10 000; LM received fees from Actelion, GlaxoSmithKline <€10 000 each; CA received fees from Pfizer, Actelion, GlaxoSmithKline <€10 000 each; JA received fees from Actelion, Sanofi and Roche <€10 000 each; PJ received fees from Actelion and Bayer <€10 000 each; PiCl works as an independent statistician.
Patient consent Obtained.
Ethics approval Comité de Protection des Personnes of Lille University Hospital and the Health Authority for the Safety of Health Products (Afssaps: Agence française de sécurité sanitaire des produits de santé). The ClinicalTrials.gov identifier was NCT01295736.
Provenance and peer review Not commissioned; externally peer reviewed.