Diagnosis and management of hypertension in pregnancy: summary of updated NICE guidance
BMJ 2019; 366 doi: https://doi.org/10.1136/bmj.l5119 (Published 09 September 2019) Cite this as: BMJ 2019;366:l5119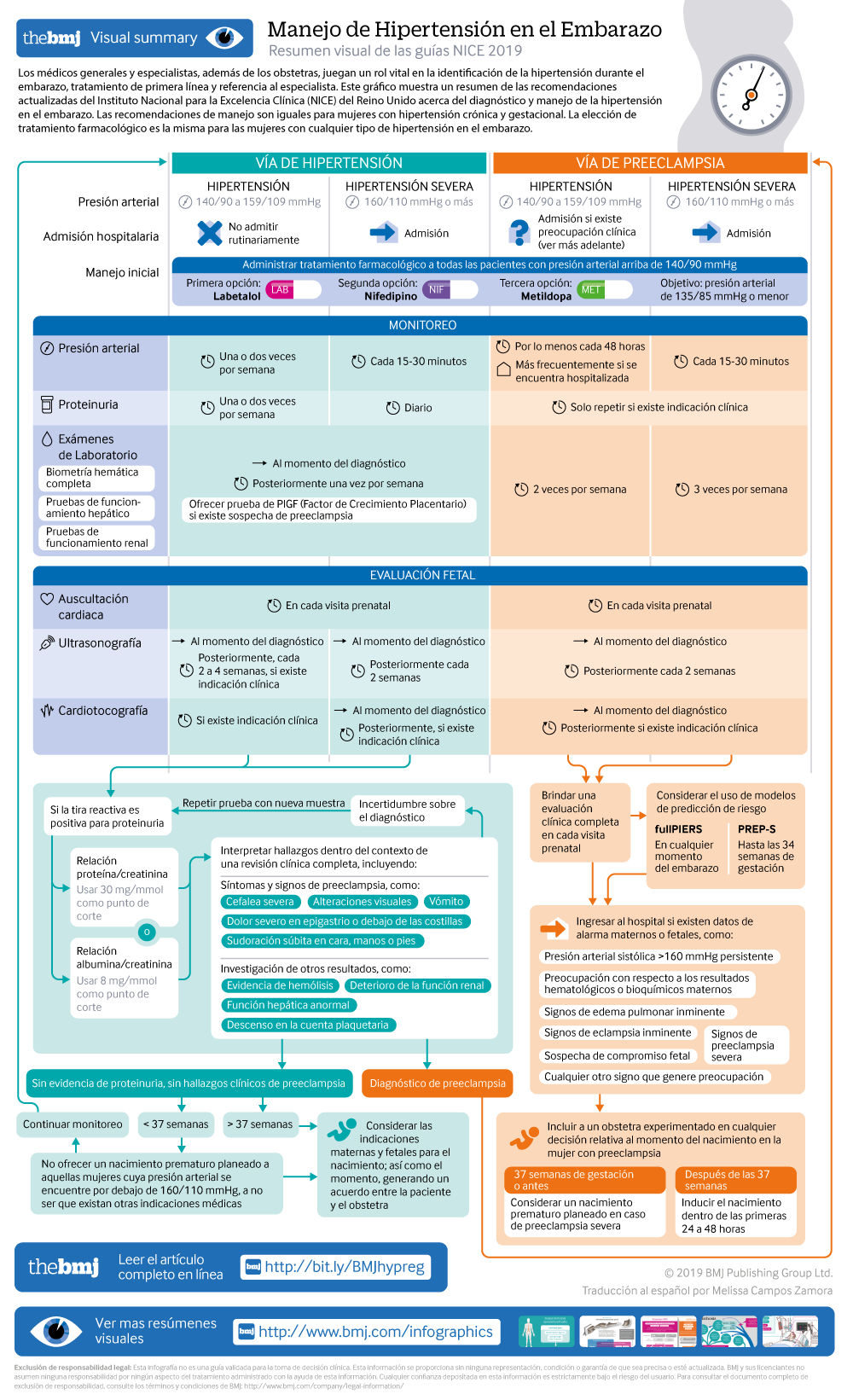
Visual summary: Resumen visual
Spanish version: Versión en español
What you need to know
Hypertension affects about 10% of pregnant women, including those with pre-existing hypertension, chronic hypertension that is first diagnosed during pregnancy, and hypertension related to pregnancy (gestational hypertension and pre-eclampsia)
Target blood pressure during the antenatal period should be 135/85 mm Hg for women with hypertension during pregnancy
Hypertension during pregnancy is associated with an increased risk of hypertension and cardiovascular disorders in later life. Women should be offered appropriate lifestyle and dietary advice to minimise this risk
Hypertension in pregnancy is a common condition, affecting about 10% of pregnant women. This includes women with chronic hypertension—which may be diagnosed before pregnancy or in the early stages of pregnancy (<20 weeks’ gestation)—and women with hypertension related to pregnancy (gestational hypertension and pre-eclampsia) (see box 1). If not identified and treated, hypertension can lead to adverse events for both the woman and her baby, including increased risk of maternal stroke, lower birth weight, and increased risk of the baby requiring neonatal intensive care.
Definitions for hypertensive disorders of pregnancy
Chronic hypertension—Hypertension that is present at the booking visit or before 20 weeks’ gestation, or if the woman is already taking antihypertensive medication when starting maternity care. It can be primary or secondary in aetiology
Gestational hypertension—New hypertension presenting after 20 weeks of pregnancy without significant proteinuria
Pre-eclampsia—New onset hypertension (>140 mm Hg systolic or >90 mm Hg diastolic) after 20 weeks of pregnancy and the coexistence of one or both of the following new-onset conditions:
Proteinuria (urine protein:creatinine ratio ≥30 mg/mmol, or albumin:creatinine ratio ≥8 mg/mmol, or ≥1 g/L [2+] on dipstick testing)
Other maternal organ dysfunction, including features such as renal or liver involvement, neurological or haematological complications, or uteroplacental dysfunction (such as fetal growth restriction, abnormal umbilical artery Doppler waveform analysis, or stillbirth)
General practitioners and specialists other than obstetricians play a vital role in the identification of hypertension during pregnancy, first line management, and appropriate referral to specialist care. Women with pre-existing (chronic) hypertension may require pre-pregnancy counselling from their primary or secondary care team, modifications to their usual treatment, and referral to specialist care. Women are likely to have shared care between specialists and non-specialists throughout their pregnancy, meaning that GPs need to be aware of current blood pressure targets, suitable medication, and thresholds for urgent referral to specialist care. Furthermore, hypertensive disorders of pregnancy are known to predispose women to ongoing hypertension and associated cardiovascular morbidity in later life. The primary care team plays a crucial role in risk reduction and surveillance for these conditions. It is therefore vital that all healthcare professionals have an understanding of the optimal management of hypertension during pregnancy and the postpartum period.
This article summarises the updated recommendations from the National Institute for Health and Care Excellence (NICE) on the diagnosis and management of hypertension in pregnancy.1
What's new in this guidance?
Initiation of antihypertensive medication is now recommended for women with a blood pressure measurement of 140/90 mm Hg
Target blood pressure for those taking antihypertensive medication is now 135/85 mm Hg
Categories of hypertension have now been simplified to “hypertension” and “severe hypertension” (rather than mild, moderate, and severe)
24 hour urine collection is no longer recommended for routine quantification of proteinuria during pregnancy
Hospital admission is no longer recommended for every woman with pre-eclampsia—risk assessment should be carried out on an individual basis to determine place of care
Pharmacological therapy for hypertension in the postnatal period now reflects stepped treatment recommended for adults, adapted for women who are breastfeeding
Estimates for the likelihood of recurrent hypertensive disorders in future pregnancies and of long term cardiovascular disease are provided
Recommendations
NICE recommendations are based on systematic reviews of best available evidence and explicit consideration of cost effectiveness. When minimal evidence is available, recommendations are based on the guideline committee’s experience and opinion of what constitutes good practice. Evidence levels for the recommendations are given in italic in square brackets.
Treatment of chronic hypertension
For women with chronic hypertension, recommended diet and lifestyle advice have been brought in line with that given to non-pregnant individuals. However, the choice of anti-hypertensive drugs is different during pregnancy, because of the need to consider the effects of the drug on the fetus. No specific medication was recommended in the previous version of the guideline, but labetalol, nifedipine, and methyldopa are now specified as suitable options to discuss with women for use in pregnancy. New evidence was identified to provide guidance on blood pressure targets during pregnancy, and the target has now been amended to 135/85 mm Hg (reduced from the previous guidance of 150/100 mm Hg), also reflecting evidence informing the management of hypertension in adults.
In addition to the new recommendations, NICE diagnostic guidance DG23 has been published since the previous guideline, and provides guidance on the use of placental growth factor (PlGF)-based testing.2 This offers an additional diagnostic test to rule out pre-eclampsia in women with suspected pre-eclampsia (including those at increased risk of developing it, such as women with chronic hypertension or gestational hypertension), and so a link has been included in the updated guideline.
The recommendations are summarised below.
Offer pregnant women with chronic hypertension advice on:
Weight management
Exercise
Healthy eating
Lowering the amount of salt in their diet.
Provide this advice in line with the NICE guideline on hypertension in adults: diagnosis and treatment3
[Based on the experience and opinion of the Guideline Committee (GC)]
Continue with existing antihypertensive treatment if it is safe in pregnancy, or switch to an alternative treatment, unless:
Sustained systolic blood pressure is <110 mm Hg or
Sustained diastolic blood pressure is <70 mm Hg or
The woman has symptomatic hypotension.
[Based on the experience and opinion of the GC]
Offer antihypertensive treatment to pregnant women who have chronic hypertension and who are not already on treatment if they have:
Sustained systolic blood pressure ≥140 mm Hg or
Sustained diastolic blood pressure ≥90 mm Hg.
[Based on very low to moderate quality evidence and the experience and opinion of the GC]
When using antihypertensive treatment in pregnancy, aim for a target blood pressure of 135/85 mm Hg. [Based on very low to moderate quality evidence and the experience and opinion of the GC]
Consider labetalol to treat chronic hypertension in pregnant women. Consider nifedipine for women in whom labetalol is not suitable, or methyldopa if both labetalol and nifedipine are not suitable. Base the choice on any pre-existing treatment, side effect profiles, risks (including fetal effects), and the woman’s preference. [Based on very low quality evidence and the experience and opinion of the GC]
Offer pregnant women with chronic hypertension aspirin 75-150 mg once daily from 12 weeks. [Based on very low to high quality evidence and the experience and opinion of the GC]
Offer placental growth factor (PlGF)-based testing to help rule out pre-eclampsia between 20 weeks and up to 35 weeks of pregnancy, if women with chronic hypertension are suspected of developing pre-eclampsia.
Management of gestational hypertension
Management of gestational hypertension requires regular monitoring, to ensure that blood pressure control is maintained and that there is not progression to pre-eclampsia. The evidence for the type and frequency of monitoring was reviewed as part of this update, and the recommendations amended. The blood pressure target has been reduced to 135/85 mm Hg (in line with that for chronic hypertension), and the drug choices aligned to those used in chronic hypertension to simplify management for clinicians.
The recommendations are summarised in the infographic [based on very low to moderate quality evidence and the experience and opinion of the GC]
Assessment of proteinuria in hypertensive disorders of pregnancy
Proteinuria is one of the key features of pre-eclampsia and should be assessed at each antenatal visit alongside blood pressure monitoring (see related NICE guidance on antenatal care for uncomplicated pregnancies4). The updated recommendations stress that proteinuria measurements should always be interpreted alongside a full clinical review—to highlight that women may develop pre-eclampsia in the absence of proteinuria, and that there may be value in repeating a measurement if there is doubt over the diagnosis of pre-eclampsia.
Previous NICE guidelines recommended that proteinuria was assessed using a 24-hour urine collection or a spot urinary protein:creatinine ratio. The updated guideline assessed the evidence for the accuracy of protein:creatinine ratio and of the alternative test albumin:creatinine ratio and found both to have high specificity and sensitivity, meaning they can be used instead of 24-hour urine collection, which is no longer recommended.
The recommendations are summarised below.
Interpret proteinuria measurements for pregnant women in the context of a full clinical review of symptoms, signs, and other investigations for pre-eclampsia. [Based on the experience and opinion of the GC]
Use an automated reagent-strip reading device for dipstick screening for proteinuria in pregnant women in secondary care settings. [Based on high quality evidence and the experience and opinion of the GC]
If dipstick screening is positive (1+ or more) use albumin:creatinine ratio or protein:creatinine ratio to quantify proteinuria in pregnant women. [Based on very low to low quality evidence and the experience and opinion of the GC]
Do not use first morning urine void to quantify proteinuria in pregnant women. [Based on very low quality evidence]
Do not routinely use 24-hour urine collection to quantify proteinuria in pregnant women.
If using protein:creatinine ratio to quantify proteinuria in pregnant women:
Use 30 mg/mmol as a threshold for significant proteinuria
If the result is ≥30 mg/mmol and there is still uncertainty about the diagnosis of pre-eclampsia, consider re-testing on a new sample, alongside clinical review.
[Based on very low quality evidence and the experience and opinion of the GC]
If using albumin:creatinine ratio as an alternative to diagnose pre-eclampsia in pregnant women with hypertension:
Use 8 mg/mmol as a diagnostic threshold
If the result is ≥8 mg/mmol and there is still uncertainty about the diagnosis of pre-eclampsia, consider re-testing on a new sample alongside clinical review.
[Based on low quality evidence and the experience and opinion of the GC]
Pre-eclampsia
Pre-eclampsia can be associated with severe complications for a woman and her baby, so appropriate risk assessment and management is critical. The updated guidance uses the same blood pressure target and treatment choices as for chronic and gestational hypertension, simplifying management for the clinician, but no longer recommends that all women with pre-eclampsia be admitted to hospital as evidence for this approach was lacking. Instead, the guideline provides more information on the features which may indicate more severe disease requiring admission and provides information on new risk prediction models that may help identify women at risk of severe complications.
Assessment
Carry out a full clinical assessment at each antenatal appointment for women with pre-eclampsia and offer admission to hospital for surveillance and any interventions needed if there are concerns for the wellbeing of the woman or baby. Concerns could include any of the following:
Sustained systolic blood pressure ≥160 mm Hg
Any maternal biochemical or haematological investigations that cause concern, such as a new and persistent
Rise in creatinine concentration (≥90 μmol/L, ≥1 mg/100 mL) or
Rise in alanine transaminase (>70 IU/L or twice upper limit of normal range) or
Fall in platelet count (<150 000/μL)
Signs of impending eclampsia
Signs of impending pulmonary oedema
Other signs of severe pre-eclampsia
Suspected fetal compromise
Any other clinical signs that cause concern.
[Based on the experience and opinion of the GC]
Consider using either the fullPIERS or PREP-S validated risk prediction models to help guide decisions about the most appropriate place of care (such as the need for in utero transfer) and thresholds for intervention. [Based on moderate to high quality evidence]
When using a risk prediction model, take into account:
fullPIERS is intended for use at any time during pregnancy
PREP-S is intended for use only up to 34 weeks of pregnancy
fullPIERS and PREP-S models do not predict outcomes for babies.
[Based on moderate to high quality evidence]
Management
Recommendations for management of pre-eclampsia are described in the infographic [based on very low to moderate quality evidence and the experience and opinion of the GC]
Planned early birth
Guidance on the indications and optimum timing (box 2) for birth in women with pre-eclampsia has also been updated.
Timing of birth in women with pre-eclampsia
Before 34 weeks’ pregnancy—Continue surveillance unless there are indications for planned early birth (see recommendation). Offer intravenous magnesium sulfate and a course of antenatal corticosteroids in line with the NICE guideline on preterm labour and birth5
From 34 to 36+6 weeks—Continue surveillance unless there are indications for planned early birth (see recommendation). When considering planned early birth, take into account the woman’s and baby’s condition, risk factors (such as maternal comorbidities, multi-fetal pregnancy), and availability of neonatal unit beds. Consider a course of antenatal corticosteroids in line with the NICE guideline on preterm labour and birth5
From 37 weeks onwards—Initiate birth within 24-48 hours.
[Based on the experience and opinion of the GC]
Record maternal and fetal thresholds for planned early birth before 37 weeks in women with pre-eclampsia. Thresholds for considering planned early birth could include (but are not limited to) any of the following known features of severe pre-eclampsia:
Inability to control maternal blood pressure despite using three or more classes of antihypertensives in appropriate doses
Maternal pulse oximetry <90%
Progressive deterioration in liver function, renal function, haemolysis, or platelet count
Ongoing neurological features, such as severe intractable headache, repeated visual scotomata, or eclampsia
Placental abruption
Reversed end-diastolic flow seen in umbilical artery Doppler velocimetry, a non-reassuring cardiotocograph, or stillbirth.
Other features not listed above may also be considered in the decision to plan early birth.
[Based on the experience and opinion of the GC]
Involve a senior obstetrician in any decisions on timing of birth for women with pre-eclampsia. [Based on the experience and opinion of the GC]
Discuss with the anaesthetic team if birth is planned in a woman with pre-eclampsia. [Based on the experience and opinion of the GC]
Discuss with the neonatal team if birth is planned in a woman with pre-eclampsia, and neonatal complications are anticipated. [Based on the experience and opinion of the GC]
Offer intravenous magnesium sulfate and a course of antenatal corticosteroids if indicated, if early birth is planned for women with preterm pre-eclampsia, in line with the NICE guideline on preterm labour and birth.5
Postnatal care for women with hypertension during pregnancy
Many women with hypertension during pregnancy will require antihypertensive treatment in the postnatal period, although the duration of treatment required will vary. Selection of an appropriate antihypertensive depends on the efficacy, safety, and tolerability of the different medications. To improve adherence, preparations with once daily use that are compatible with breast feeding are recommended. The recommendations were updated, based on the NICE guideline for the management of hypertension in adults,3 adapted to support breastfeeding in women who may choose to breastfeed and to minimise the chance of women choosing not to breastfeed because of their medication.
Advise women with hypertension who wish to breastfeed that their treatment can be adapted to accommodate breastfeeding and that the need to take antihypertensive medication does not prevent them from breastfeeding. [Based on the experience and opinion of the GC]
Explain to women with hypertension who wish to breastfeed that:
Antihypertensive medicines can pass into breast milk
Most antihypertensive medicines taken while breastfeeding only lead to very low levels in breast milk, so the amounts taken in by babies are very small and would be unlikely to have any clinical effect
Most medicines are not tested in pregnant or breastfeeding women, so disclaimers in the manufacturer’s information are not because of any specific safety concerns or evidence of harm.
[Based on very low to moderate quality evidence and the experience and opinion of the GC]
Make decisions on treatment together with the woman, based on her preferences. [Based on the experience and opinion of the GC]
As antihypertensive agents have the potential to transfer into breast milk:
Consider monitoring the blood pressure of babies, especially those born preterm, who have symptoms of low blood pressure for the first few weeks
When women are discharged home, advise them to monitor their babies for drowsiness, lethargy, pallor, cold peripheries, or poor feeding.
[Based on the experience and opinion of the GC]
Offer enalapril to treat hypertension in women during the postnatal period, with appropriate monitoring of maternal renal function and maternal serum potassium. [Based on the experience and opinion of the GC]
For women of black African or Caribbean family origin with hypertension during the postnatal period, consider antihypertensive treatment with:
Nifedipine or
Amlodipine if the woman has previously used this successfully to control her blood pressure.
[Based on very low to low quality evidence and the experience and opinion of the GC]
For women with hypertension in the postnatal period, if blood pressure is not controlled with a single medicine consider a combination of nifedipine (or amlodipine) and enalapril. If this combination is not tolerated or is ineffective, consider:
Adding atenolol or labetalol to the combination treatment or
Swapping one of the medicines already being used for atenolol or labetalol.
[Based on the experience and opinion of the GC]
When treating women with antihypertensive medication during the postnatal period, use medicines that are taken once daily when possible. [Based on the experience and opinion of the GC]
When possible, avoid using diuretics or angiotensin receptor blockers to treat hypertension in women in the postnatal period who are breastfeeding or expressing milk. [Based on the experience and opinion of the GC]
Treat women with hypertension in the postnatal period who are not breastfeeding and who are not planning to breastfeed in line with the NICE guideline on hypertension in adults.3 [Based on the experience and opinion of the GC]
Long term consequences of hypertension during pregnancy
The occurrence of hypertension during one pregnancy is known to predispose women to hypertension in the future—with an increased likelihood of recurrence of hypertensive disorders of pregnancy in future pregnancies and of long term hypertension in later life.
Precisely quantifying the likelihood of recurrence during pregnancy is challenging, but the updated guidance provides some estimates of how likely hypertensive disorders are to recur (table 1). Advise women with hypertensive disorders of pregnancy that the overall risk of recurrence in future pregnancies is approximately 1 in 5.
Prevalence of hypertensive disorder in a future pregnancy in women with hypertension in previous or current pregnancy [Based on very low to high quality evidence and the experience and opinion of the GC]
In addition, hypertensive disorders during pregnancy are known to be associated with an increased likelihood of hypertension, and associated cardiovascular morbidity, in later life. The updated guideline provides estimates of this increase in likelihood for women with hypertensive disorders during pregnancy, to enable them to modify their lifestyle accordingly.
Advise women who have had a hypertensive disorder of pregnancy that this is associated with an increased risk of hypertension and cardiovascular disease in later life (see table 2). [Based on moderate to high quality evidence and the experience and opinion of the GC]
Advise women who have had a hypertensive disorder of pregnancy to discuss how to reduce their risk of cardiovascular disease, including hypertensive disorders, with their GP or specialist. This may include:
In women who have had pre-eclampsia or hypertension with early birth before 34 weeks consider pre-pregnancy counselling to discuss possible risks of recurrent hypertensive disorders of pregnancy and how to lower them for any future pregnancies. [Based on the experience and opinion of the GC]
Relative risk* of future cardiovascular morbidity in women with hypertension in previous or current pregnancy
Implementation
A patient decision aid has been developed to support implementation of this guideline and is available at https://action-on-pre-eclampsia.org.uk/public-area/high-blood-pressure-in-pregnancy/#resources.
PREP and fullPIERS clinical prediction tools are freely available online
Future research
Further research is needed on the efficacy and safety of antihypertensive agents during pregnancy and the postnatal period—including the comparative efficacy of different antihypertensives to treat chronic hypertension, the neonatal effects of β blockers and mixed α and β blockers, and the efficacy of different antihypertensives in the postnatal period.
Two areas of antenatal care were prioritised for future research—to assess whether inpatient care is associated with better outcomes for women with pre-eclampsia, and to establish the optimal fetal monitoring strategy to identify infants that are small for gestational age.
Future research should concentrate on the efficacy of interventions to reduce the risk of recurrence of hypertension in future pregnancies, and the risk of long term cardiovascular complications.
Guidelines into practice
Do you refer women with chronic hypertension to a specialist in hypertensive disorders of pregnancy for pre-pregnancy advice?
Do you stop ACE inhibitors or angiotensin II receptor blockers within two days of notification of pregnancy?
Do you provide information for postnatal women after pregnancy hypertension on long term cardiovascular risk and interventions to reduce that risk?
How women with lived experience were involved in the creation of this article
Committee members involved in this guideline update included lay members who contributed to the formulation of the recommendations summarised here.
Further information on the guidance
The guideline update was developed using the methods described in Developing NICE guidelines: the manual, 2014 (https://www.nice.org.uk/media/default/about/what-we-do/our-programmes/developing-nice-guidelines-the-manual.pdf). Systematic literature searches were undertaken to identify all published clinical evidence and health economic evidence relevant to the review questions. The guideline committee comprised healthcare professionals and lay members, who considered the evidence identified and drafted recommendations on the basis of the evidence and the expertise and opinion of the committee. Draft recommendations were subject to stakeholder consultation and revision before publication of the final guideline.
Other details
This guideline has been published by NICE and is available at https://www.nice.org.uk/guidance/ng133.
Quick reference guides are being developed by NICE and will be available at https://www.nice.org.uk/guidance/ng133.
Acknowledgments
The members of the guideline committee were (in alphabetical order) Philip Barclay, Sarah Beswick, Lucy Chappell, Alena Chong, Maria Clark, Sarah Findlay, Sarah Fishburn (chair), Christine Harding, Pramod Mainie, Maryam Parisaei, Lisa Smith, Mark Tighe, Ashifa Trivedi, and Pensee Wu.
The members of the National Guideline Alliance team were (in alphabetical order) Offiong Ani, Hilary Eadon, Louise Geneen, Eva Gonzalez-Viana, Matthew Prettyjohns, Tim Reeves, and Katie Webster.
Footnotes
Contributors: All authors contributed to the initial draft of this article, helped revise the manuscript, and approved the final version for publication.
Funding: The National Guideline Alliance was commissioned and funded by the National Institute for Health and Care Excellence to develop this guideline and write this BMJ summary.
Disclaimer: The guideline referred to in this article was produced by the National Guideline Alliance for the National Institute for Health and Care Excellence (NICE). The views expressed in this article are those of the authors and not necessarily those of NICE.
Competing interests: We declare the following interests based on NICE's policy on conflicts of interests (https://www.nice.org.uk/Media/Default/About/Who-we-are/Policies-and-procedures/declaration-of-interests-policy.pdf): SF has received funding from NICE, National Institute for Health Research, Royal College of Obstetricians and Gynaecology and Mott MacDonald. SCF has received funding from the BMJ. The authors’ full statements can be viewed at https://www.nice.org.uk/guidance/ng133/documents/register-of-interests-2.

